Oxidation and Reduction Reactions
advertisement

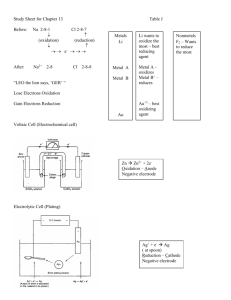
Oxidation and Reduction Reactions and Electrochemistry “The Ubiquitous Electron” Redox and Iron in your Body Types of Reactions 1. 2. Ions or molecules react w/ no apparent change in electronic structure (ex. Double displacement) Ions or atoms undergo changes of electronic structure, the way e- transfer or the way atoms share e- changes. Oxidation- Reduction Reaction Definition: Chemical change that occurs when electrons are transferred between reactants All oxidation reactions are accompanied by reduction reactions Important: in the corrosion of metals, sources of energy, life processes Oxidation Part of the redox rxn in which electrons are removed or apparently removed from an atom (loss of electrons atom gets more positively charged) Movie Reduction Part of the redox rxn in which electrons are added or apparently added to an atom (gain of electrons atoms get more negatively charged) Movie OIL RIG Oxidation Is Losing Reduction Is Gaining LEO the lion goes GER Loss of Electrons in Oxidation Gain of Electrons in Reduction Ionization or Solvation = the process of surrounding solute particles with solvent particles to form a solution Video “like dissolves like” Net Ionic Equations When reactions take place in water chemists write the equation in ionic form (particles ionize – break into their ions in water) Chemists only write down the ions that take part in the reaction Spectator ions- ions that aren’t involved in the reaction (chemists don’t write these) Makes rxn easier to balance Cu + NO3-1 Cu+2 + NO Show chemistry connections video: 7:36 minutes into video, found in redox folder Rules for Assigning Oxidation Numbers: Use oxidation numbers (charges on atoms) to determine which atom underwent reduction and which atom underwent oxidation Rules: 1. The oxidation number for any free element is 0 (zero). Also any diatomic molecule is 0 (zero) H2, O2, I2, Cl2, F2, N2, Br2 Fe = 0 charge O2 = 0 charge 2. The oxidation number of any monoatomic ion is equal to the charge written on the ion. Na +1 = +1 Cl-1 = -1 3. Oxidation number of hydrogen in most of its compounds is +1 (except for LiH then H is –1) +1 Ex. HCl 4. Oxidation # of oxygen in most of its compounds is –2.(except peroxides= -1) -2 Ex. H2O -1 Ex. H2O2 5. Sum of the oxidation numbers of all of the atoms must equal the apparent charge of that particle. Ex. H2SO4 -zero charge +1 ? -2 H2SO4 +2 +6-8=0 S= +6 Ex. NO3 –1 ? + -2(3) = -1 +5 + (-6) = -1 N= +5 6. Group 1 +1 Group 2 +2 Aluminum & Boron +3 Group 17 -1 Ex. KMnO4 K= +1 Mn = +7 O = -2 Page 174 #67, 69 Identifying redox, chemistry connections 11:29 minutes in Identifying Redox Reactions First, figure out the oxidation numbers of all elements in the reaction If oxidation number changes as you move from reactants to products it is REDOX. This is REDOX, Mg- loss e(oxidation), H –gained e-(reduction) This is NOT REDOX P 618 in modern chem- #2, 15 Oxidizing & Reducing Agents Think of these agents as “causers” of redox rxns Look at reactants Some substances are better oxidizing or reducing agents Reducing Agents: substance that donates the electron (contains the atoms that are oxidized- or loss the e-) • Causes the reduction to occur Oxidizing Agent: substance that gains the e- (contains the atoms that are reduced or gains e-) • Causes oxidation to occur Ex. 0 0 +3 -2 4Al + 3O2 2Al2O3 Al- lost e- , oxidized -reducing agent O- gained e-, reduced -O2 is the oxidizing agent Balancing Redox Reactions --Half Reaction Method Half Reaction: equation that shows just the oxidation or reduction part of the rxn. In balancing we balance each of the half rxns first, then add them together & reduce Steps: 1. 2. 3. 4. Place oxidation #’s on everything after it is in the net ionic form. ID the oxidation ½ rxn and the reduction ½ rxn Write out the ½ rxns. Balance the atoms by placing coefficients in front of the atoms except for H and O Ex. Cl2 Cl-1 become Cl2 2Cl- 5. Place the # of electrons lost on the product side of oxidation ½ rxn, place # of electrons gained on reactant side of reduction ½ rxn 6. To balance hydrogens and oxygens: Acidic soln: add H+ & H2O Basic soln: add OH- & H2O 7. 8. Balance the charges (# e- lost must equal # e- gained) by using a least common multiple ( multiply the whole ½ rxn) Add two ½ rxns together and reduce if necessary. Chemistry connections- balancing with blood alcohol tests (21:00-26:00) Electrochemistry Movie Because redox reactions involve electron transfer, the release or absorption of energy can occur in the form of electrical energy rather than heat Electrochemistry is the branch of chemistry that deals w/ electricity related applications of redox reactions Electrochemical Process Conversion of chemical to electrical energy Ex. Flashlight batteries, biological systems, electroplating If the substance that is oxidized is separated from the substance that is reduced you get an energy transfer of electrical energy instead of heat Electrons can be transferred from one side to the other through a connecting wire Electric current moves in a circuit (while the electrons are being balanced by the movement of ions in solution) Part of a Cell: Electrodes: – Conductor in a circuit that carries electrons from one substance to another – Anodes: electrode where oxidation occurs, anions (-) are attracted to this when they are oxidized by losing electrons (the positive electrode) – Cathode: electrode where reduction occurs, cations (+) are attracted to this when they are reduced by gaining electrons (negative electrode) Salt Bridge: – Porous partition that separates the 2 half reactions – Contains a conducting solution that allows the passage of ions from one compartment to the other w/ out mixing the solutions in the half reactions Half Cell: – Part of the voltaic cell in which either oxidation or reduction occurs – The two half cells together make a complete electrochemical cell Ex. Oxidation half cell – Zn Zn+2 + 2 e• (zinc rod in zinc sulfate) Reduction half cell – Cu+2 + 2e- Cu • (copper rod in copper sulfate) Complete Cell Notation Anode electrode |anode solution || cathode solution |cathode electrode (the double line || represents the salt bridge) Ex. Zn (s) | Zn +2 (aq) || Cu+2 (aq) | Cu (s) e- e- e- e- Anode-positive electrode, oxid. occurs Zn rod ZnSO4 Salt bridge Cu rod CuSO4 Zn(s) |ZnSO4(aq)||CuSO4 (aq)| Cu (s) Cathodeneg. electrode, red. occurs Fuel Cell Type of Cells Dry Cell: voltaic cell in which the electrolyte (conducting solution) is a paste – – – – Generates direct current by converting chemical to electrical energy by a spontaneous redox reaction Also called galvanic cells or voltaic cells Ex. Batteries (zinc-carbon, alkaline, mercury) Ex. Flashlight battery (zinc-carbon) • Zinc container (anode) filled w/ a moist paste (salt paste) made of MnO2, ZnCl2, NH4Cl and water w/ a graphite rod (cathode) embedded into it – Alkaline batteries (do not have a carbon rod cathode which allows them to be smaller- uses a graphite/ MnO2 mix) – Mercury (cathode is HgO/carbon mix) – Lead storage batteries • Group of cells that are connected together • Can be recharged (use in a car) • Ex. 12 V battery- 6 voltaic cells connected together – – – – Each cell contains 2 lead electrodes or grids Anode- grid packed w/ spongy lead Cathode – grid packed w/ PbO2 Immersed in 5M H2SO4 • Recharging occurs whenever the car is running • Doesn’t last forever- byproduct PbSO4 falls from electrodes and collects on bottom (loses too much lead) – Fuel Cells • A voltaic cell in which the reactants are being continuously supplied and the product are being continuously removed • A fuel substance undergoes oxidation, from which electrical energy is obtained continuously • No recharging, no pollution • Ex. H-O cell: submarines, military vehicles, Apollo Electrical Potential In a voltaic cell, the oxidizing agent at the cathode pulls the electrons through the wire away from the reducing agent at the anode The “pull” on the electrons is called the electric potential Electrical potential is measured in volts (V) Electrode potential: The potential difference measure across the complete voltaic cell is easily measured It equals the sum of the electrode potentials for each of the two half-reactions The individual electrode potential for a halfreaction cannot be measured directly, but it can be measured by connecting to a standard half-cell as a reference (we use a Hydrogen electrode that is in a 1.0M acidic solution at 1 atm and 25 C) Standard Reduction Potentials (p. 796-book) Electrode potentials are always written as reductions The more negative the voltage oxidation (stronger reducing agent) The more positive the voltage reduction (stronger oxidizing agent) Standard Cell Potential (E° cell) Use this formula: E°cell = E°reduction - E°oxidation or E°cell = E°cathode - E°anode A spontaneous reaction will have positive value for E° cell Zn (s) | Zn +2 (aq) || Cu+2 (aq) | Cu (s) Oxidation: Zn+2 + 2 e- Zn – E°Zn +2 = -.76 V Reduction: Cu +2 + 2e- Cu – E°Cu+2 = .34V E°cell = E°reduction - E°oxidation =.34V - (-.76V) =1.10V Cu+2 + Zn Cu + Zn+2 Zn (s) | Zn +2 (aq) || Fe+2 (aq) | Fe (s) (anode) (cathode) Oxidation: Zn+2 + 2 e- Zn – E°Zn +2 = -.76 V Reduction: Fe +2 + 2e- Fe – E°Fe+2 = -.44V E°cell = E°reduction - E°oxidation =-.44V - (-.76V) =.32V Fe+2 + Zn Fe + Zn+2 Practice Mn| Mn +2 || Br2 | BrH2C2O4| CO2 || MnO4-1 | Mn+2 Ni | Ni +2 || Hg2+2 | Hg Cu | Cu+2 || Ag+1 | Ag Pb| Pb +2 || Cl2 | Cl- Mn| Mn +2 || Br2 | Br– – Ecell= 1.07-(-1.18)= 2.25 V Br2 +Mn Mn+2 + 2Br- H2C2O4| CO2 || MnO4-1 | Mn+2 – E cell= 1.51- (-.49) = 2.00 V – 2 MnO4- +6 H+ + 5 H2C2O4 2Mn+2 + 8H2O + 10 CO2 Ni | Ni +2 || Hg2+2 | Hg – 1.04 V – Ni + Hg2 +2 Ni +2 + 2Hg Cu | Cu+2 || Ag+1 | Ag – .46 V – Cu + 2 Ag+ Cu+2 + 2 Ag Pb| Pb +2 || Cl2 | Cl– – `1.49V Pb +Cl2 Pb+2 + 2Cl- Video How its made nails Corrision Pics Redox/ Electrochemistry Quest (anode song) Redox – – – – – oxidation #’s ID if redox or not Oxidizing or reducing agent (strengths) Balancing- set up ½ rxns Balancing oxygens/hydrogens • Acids (add H+ and H20) • Bases (add OH- and H20) Electrochem – What is an electrochemical cell – Example – Parts: anode, cathode, salt bridge, what each part does) – Standard cell potential (getting the voltage and write equation)