Inhalation Anesthesia 2012-1
advertisement

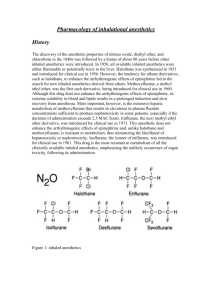
Clinical Pharmacology of Inhaled Anesthetics Dr. Greg Bryson Dept of Anesthesiology The Ottawa Hospital 2012.09.27 Objectives I • • • • • • • Chemical structure Structure - function relationships Physiochemical properties Definition of MAC Factors which affect MAC Mechanism of action Uptake and Distribution Objectives II • Fa/Fi curves, and factors which affect them • Physiological effects of inhalation anesthetics: – Cardiovascular system – Respiratory system – Central nervous system – Neuromuscular junction – Others • Metabolism/toxicity of inhalation anesthetics The reality • • • • • • You use these drugs every day If you don’t know them – no one else does None of this stuff is “new” All of it is in the textbooks Some of it is useful – some is “on the exam” I can’t cover all of it in 3 hours Greg’s goals for this lecture • • • • • Inflict my view of what you should know Put this in a clinical (read: useful) context Explain that which needs explaining Leave the memory work to you Take my daughter to cross country practice Reference material • Miller 7th Edition – Chapter 20. Inhaled Anesthetics. Mechanisms of Action. – Chapter 21. Inhaled Anesthetics. Uptake and Distribution. – Chapter 22. Pulmonary Pharmacology. – Chapter 23. Cardiovascular Pharmacology. – Chapter 24. Inhaled Anesthetics. Metabolism and Toxicity. Chemical structure I Xe Nitrous Oxide Xenon Halothane Diethyl Ether Fun with chemistry • • • • Alkanes precipitate arrythmias Halogenation reduces flammability Fluorination reduces solubility Trifluorcarbon groups add stability Chemical structure II F Cl H F Enflurane Isoflurane Desflurane Sevoflurane Physical characteristics • Please cram the contents of table 15.1 from Barash 5th Ed the night before the exam. Take home points include: – desflurane boils at 24 OC – halothane is preserved with thymol – vapor pressures are needed for some exam questions – knowledge of blood:gas partition coefficients may actually be useful (uptake and distribution) Vapor pressure and vaporizer Fig 25-17. Miller 7th Ed Partition coefficients • Represent the relative affinity of a gas for 2 different substances (solubility) • Measured at equilibrium so partial pressures are equal, but... • The amounts of gas dissolved in each substance (concentration) aren’t equal. • The larger the number, the more soluble it is in the first substance Key Physical Properties Drug Nitrous Oxide Xenon MAC 105 71 Oil:Gas PC Blood:Gas PC 1.4 1.9 0.47 0.14 Desflurane 6.0 19 0.45 Sevoflurane 1.71 53 0.65 Enflurane *1.68 *97 1.4 Isoflurane 1.15 90 1.8 Halothane *0.74 *224 2.5 Tables 21.1 and 24.1. Miller 7th Ed * Old textbooks on my shelf Mechanism of Action Meyer-Overton Protein-interference Fig 20-2 Miller 7th Ed Mechanism of action II • Protein Receptor Hypothesis – ligand-gated ion channels (GABA, glycine, NMDA-glutamate) – voltage-gated ion channels (Na, K, Ca) – G-proteins (guanine nucleotide) – Protein kinase C • Site of action – Brain v spinal cord (amnesia v immobility) – Axonal v synaptic – Pre v post synaptic Minimum alveolar concentration • Alveolar concentration required to prevent movement in 50% of subjects • standard stimulus • represents brain concentration • consistent within and between species • Additive • Variants – BAR (1.7 – 2.0 MAC) – Awake (0.3 -0.5 MAC) Factors decreasing MAC • • • • • • • • Increasing age (6% per decade) Hypothermia Hyponatremia Hypotension (MAP<50mmHg) Pregnancy Hypoxemia (<38 mmHg) O2 content (<4.3 ml O2/dl) Metabolic acidosis • • • • • • • • Narcotics Ketamine Benzodiazepines 2 agonists LiCO3 Local anesthetics ETOH (acute) And many more... Table 15.5. Barash 5th Edition Factors increasing MAC • • • • Hyperthermia Chronic ETOH abuse Hypernatremia Increased CNS transmitters – MAOI – Amphetamine – Cocaine – Ephedrine – L-DOPA Table 15.4. Barash 5th Edition. Factors with no influence on MAC • • • • • • • • • Duration of anesthesia Sex Alkalosis PCO2 Hypertension Anemia Potassium Magnseium And others Uptake and distribution • Anesthesia depends upon brain partial pressure • Alveolar partial pressure (PA) = Pbrain • The faster PA approaches the desired level the faster the patient is anesthetized • PA is a balance between delivery of drug to the alveolus and uptake of that drug into the blood • Time for an analogy a b To induce anesthesia the bucket (PA) must be full. Unfortunately the bucket has a leak (uptake). To fill the bucket you must either (a) pour it in faster (increase delivery) or (b) slow down the leak (decrease uptake). Factors influencing uptake • • • • Solubility (blood:gas pc) Cardiac output Alveolar-venous pressure gradient For those of you who like formulae: Uptake = • Q • (PA-Pv)/BP The blood:gas pc is useful, really. • Anesthesia is related to the partial pressure of the gas in the brain. • If a drug is dissolved in blood, it isn’t available as a gas • More molecules of a soluble gas are required to saturate liquid phase before increasing partial pressure • Speed of onset/offset closely related to solubility • The lower the blood:gas pc - the faster the onset FA/FI Curves Agent Blood:Gas PC Nitrous Oxide 0.47 Desflurane 0.45 Sevoflurane 0.65 Isoflurane 1.8 Methoxyflurane 12 Factors influencing delivery • Alveolar ventilation • Breathing system – volume – fresh gas flow • Inspired partial pressure (PI) – concentration effect – second gas effect Minute ventilation and uptake Figure 21-5. Miller 7th Ed Cardiac Output and Uptake Fig 21-7. Miller 7th Ed Concentration and 2nd gas effects 54% Fig 21.3 Miller 7th Ed Concentration and 2nd Gas Effects Fig 21.4. Miller 7th Ed V/Q distribution and uptake • Ventilation < perfusion (shunt) – blood leaving shunt dilutes PA from normal lung – induction with low solubility agent will be delayed – little difference with soluble agents (slow anyway) • Ventilation > perfusion (dead space) – uptake is decreased which enhances rise in FA – may speed induction for soluble agents – less difference with low solubility agents (fast anyway) Endobronchial intubation Figs 21 – 11 and 12. Miller 7th Ed. Break Objectives II • Physiological effects of inhalation anesthetics: – Cardiovascular system – Respiratory system – Central nervous system – Neuromuscular junction – Others • Metabolism/toxicity of inhalation anesthetics Effects on organ systems • • • • • • • • Cardiovascular (Ch 23) Pulmonary (Ch 22) CNS (Ch 13) Neuromuscular Hepatic (Ch 66) Renal (Ch 45) Uterine (ch 69) Miscellaneous Inhaled anesthetics - CV system • Effect is hard to quantify • In vitro and in vivo effects often quite different – Sympathetic stimulation – Baroreceptor reflexes – Animal model vs human subject • Information provided in this lecture is a broad overview. • Chapter 23, Miller 7th Ed for details Myocardial contractility • All volatile anesthetics are direct myocardial depressants in vitro, including N2O. • Effect on circulation in vivo modified by effects on pulmonary circulation and sympathetic stimulation. • Ca++ hemostasis in sarcoplasmic reticulum • As best as we can tell, at 1 MAC anesthetics depress contractility in the following order – H = E > I = D = S. Heart rate • Effects variable and agent-specific – halothane decreases HR – Sevoflurane and enflurane neutral – Desflurane associated with transient tachycardia • occurs with rapid increases in MAC • associated with increases in serum catecholamines • similar effect may be seen with isoflurane Blood pressure • All decrease BP, except N2O • Effect caused by a combination of – Vasodilation – Myocardial depression – Decreased CNS tone • Relative contribution of each is drug dependent Cardiac output • Despite myocardial depression cardiac output is well-maintained with isoflurane and desflurane – preservation of heart rate – greater reduction in SVR – preservation of baroreceptor reflexes Systemic vascular resistance • • • • All are direct vasodilators, except N2O relax vascular smooth muscle cAMP - Ca++and/or nitric oxide involved variable effects on individual vascular beds Dysrhytmias • Halothane potentiates catecholamine-related dysrhythmias • ED50 of epinehrine producing dysrhythmias at 1.25 MAC – halothane 2.1 g•kg-1 – isoflurane 6.9 g•kg-1 – enflurane 10.9 g•kg-1 – Sevo + Des similar to isoflurane • Lidocaine doubles ED50 of epinephrine • Children somewhat more resistant Chapter 2. Stoelting P&P. 2nd Ed Chapter 5. Barash 5th ED Coronary blood flow • Isoflurane is a potent coronary vasodilator • In theory, dilation of normal coronary vessels can direct blood flow away from stenotic coronaries • Steal-prone anatomy – total occlusion of 1 major coronary vessel – collateral perfusion with 90% stenosis • In practice, doesn’t seem to be a problem Myocardial protection • Ischemic preconditioning • Volatiles appear able to replicate the effect • Activation of mitochondrial KATP channels – maintain Ca++ hemostasis – prevent mitochondrial Ca++ overload • Inhibition of adenosine 1 (A1) receptors and guanine inhibitory (Gi) proteins abolish protection • Free radical scavenging (ROS) Respiratory system • Volatile anesthetics affect all aspects of RS • With exception of bronchodilation, none good. • For all the gory details (Chapter 22. Miller 7th Ed) Bronchial musculature • Reduce vagal tone • Direct relaxation – decreased intracellular Ca++ – decreased sensitivity to Ca++ • When bronchospastic, a dose dependent reduction in Raw occurs with most agents • Exception is Xenon – Increased viscosity causes increased Raw Mucociliary function + surfactant • Volatile anesthetics decrease ciliary beat • Decreased mucus clearance • Decreased production of phosphatidylcholine – PC used to make surfactant – occurs in as little as 4 hours – reversible in 2 hours Pulmonary blood flow • Intrinsic vasodilators • Hypoxic pulmonary vasoconstriction • Intrinsic changes in HPV confounded by – changes in cardiac output – pulmonary artery pressure – position • Shunt and PO2 appear unchanged in studies of inhaled anesthetics during one lung ventilation Control of ventilation • All decrease tidal volume • Most increase frequency • Net effect is: – decrease minute ventilation – increased PaCO2 (E>D=I>S=H) • Xenon appears to do the opposite • Decreased sensitivity and response to – Hypoxia – Hypercarbia – Inspiratory and expiratory loads Lung volumes • Decreased FRC – decreased intercostal muscle activity – phasic expiratory muscle activity • Cephalad displacement of dependent diaphragm • Dependent atelectasis Enclosed Air Spaces Agent Nitrous Oxide Nitrogen Blood:Gas PC 0.47 0.014 • N20 leaves blood 34x more than N2 absorbed • Sure, other agents are more soluble but we don’t give them at 70% end-tidal concentration • distension of closed air spaces • 70% N2O will double a pneumo in 10 minutes Fig 21-13 Miller 7th Ed. Central nervous system • Increase cerebral blood flow (halo worst) • Increase ICP – related to increase in CBF • Decreased CMRO2 – uncoupled . • Decreased frequency - increased voltage on EEG • 2 MAC enflurane increases seizure activity • Decreased amplitude - increased latency on SSEP Neuromuscular function • Skeletal muscle relaxation • Potentiate NDMR • Trigger MH Hepatic • Volatile anesthetics increase hepatic arterial blood flow – decrease portal blood flow • Halothane is the exception – decreases arterial flow. • Clearance of drugs decreased in keeping with alteration in hepatic blood flow Renal • Dose-dependent decreases in – renal blood flow – glomerular filtration rate – urine output • Related to changes in CO and BP not ADH Obstetrical • N2O has no effect • Halogenated volatiles lead to dose-dependent – uterine relaxation – reductions in uterine blood flow . Metabolism of inhaled anesthetics • • • • Fairly small component of elimination Occurs at cytochrome p450 Inducible Oxidative – o-dealkylation – dehalogenation – epoxidation • Reductive – occurs only with halothane in hypoxic conditions Three determinants of metabolism • Chemical structure – ether bond – carbon-halogen bond • Hepatic enzyme activity – Cytochrome P450 (CP2E1 – Inducible • Blood concentration Metabolism of inhaled anesthetics II Agent Halothane Sevoflurane Enflurane Isoflurane Desflurane Nitrous Oxide Xenon % Metabolized 20 2-5 2.4 0.2 0.02 0.004 0.000 Table 15-1. Barash 5th Edition. Hepatic toxicity • Hepatotoxicity comes in 2 forms • mild, transient, postoperative increase in LFTs – ? due to transient hypoxia ± reductive metabolites • massive hepatic necrosis – oxidative metabolite (TFA) binds to hepatocyte – repeat exposure leads to immune-mediated necrosis • Largely a disorder of halothane (metabolism) • Some evidence for other volatiles and HCFCs Fluoride nephrotxicity • • • • Oxidative metabolism releases inorganic fluoride Toxic at serum concentrations 50 mol/l F- opposes ADH leading to polyuria Duration and intensity of exposure important – methoxyflurane 2.5 MAC-hours (renal metabolism) – enflurane 9.6 MAC-hours – sevoflurane ? Sevofluane and compound A • • • • • Reaction with alkaline, hot, C02 absorbents FGF related to heating of absorbent Baralyme > soda lime 25 – 50 ppm yields ATN in rats Rats have 20 – 30 times more -lyase – thionoacyl fluoride metabolite is toxic • Human studies have not demonstrated ATN Carbon monoxide • Interaction with dry, basic CO2 absorbent • Monday morning syndrome • All volatiles generate CO – desflurane>enflurane>isoflurane>>>sevo+hal othane – Baralyme (20% BaOH) > soda lime (CaOH, NaOH) – We use ??? Miscellaneous • N2O-related myelosupression if >12 hr exposure – inhibition of methionine-synthetase – megaloblastic anemia • Inhaled anesthetics, N2O in particular, decrease leukocyte function • Teratogenesis with prolonged exposure in rats • Increased risk (RR = 1.3) of spontaneous abortion with chronic exposure to N20 Conclusion • Damn, there is a lot to cover here. • Some overlap with other core lectures • PLEASE summarize as you read • When in doubt…blame Gproteins and cytosolic Ca