Pharmacology of inhalational anesthetics 2
advertisement
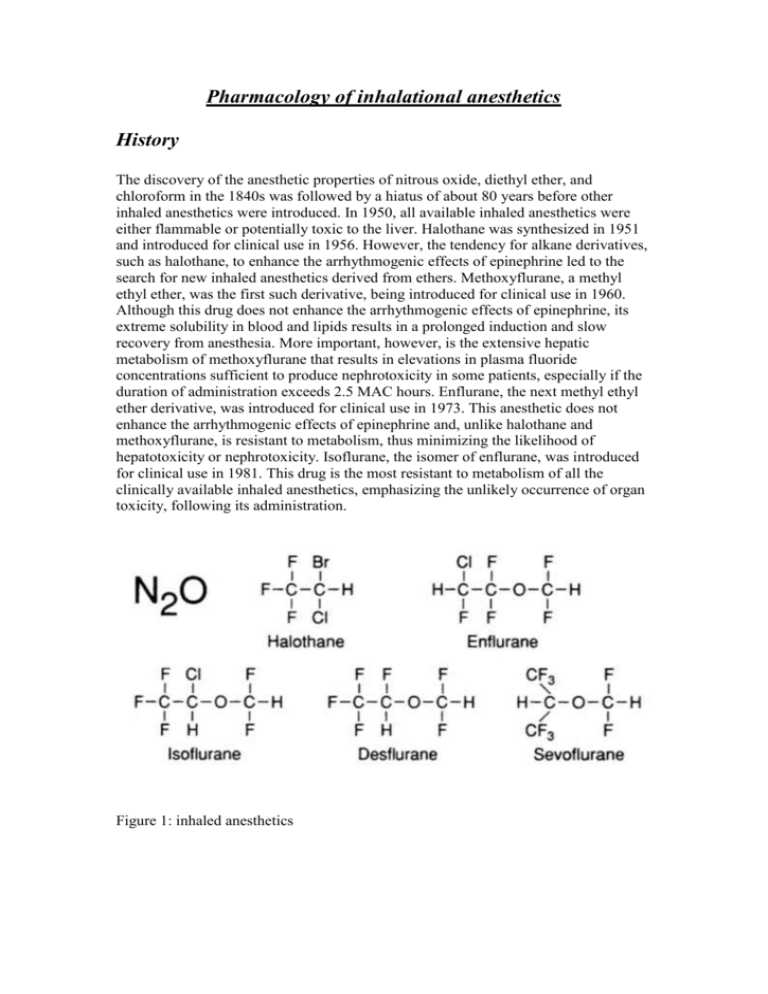
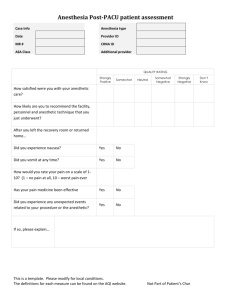
Pharmacology of inhalational anesthetics History The discovery of the anesthetic properties of nitrous oxide, diethyl ether, and chloroform in the 1840s was followed by a hiatus of about 80 years before other inhaled anesthetics were introduced. In 1950, all available inhaled anesthetics were either flammable or potentially toxic to the liver. Halothane was synthesized in 1951 and introduced for clinical use in 1956. However, the tendency for alkane derivatives, such as halothane, to enhance the arrhythmogenic effects of epinephrine led to the search for new inhaled anesthetics derived from ethers. Methoxyflurane, a methyl ethyl ether, was the first such derivative, being introduced for clinical use in 1960. Although this drug does not enhance the arrhythmogenic effects of epinephrine, its extreme solubility in blood and lipids results in a prolonged induction and slow recovery from anesthesia. More important, however, is the extensive hepatic metabolism of methoxyflurane that results in elevations in plasma fluoride concentrations sufficient to produce nephrotoxicity in some patients, especially if the duration of administration exceeds 2.5 MAC hours. Enflurane, the next methyl ethyl ether derivative, was introduced for clinical use in 1973. This anesthetic does not enhance the arrhythmogenic effects of epinephrine and, unlike halothane and methoxyflurane, is resistant to metabolism, thus minimizing the likelihood of hepatotoxicity or nephrotoxicity. Isoflurane, the isomer of enflurane, was introduced for clinical use in 1981. This drug is the most resistant to metabolism of all the clinically available inhaled anesthetics, emphasizing the unlikely occurrence of organ toxicity, following its administration. Figure 1: inhaled anesthetics Physical & chemical properties of inhalational agents (table 1 & figure 1) Nitrous Oxide Nitrous oxide is a low-molecular-weight, odorless to sweet-smelling, nonflammable gas of low potency and poor blood solubility (0.46) that is most commonly administered in combination with opioids or volatile anesthetics to produce general anesthesia. Although nitrous oxide is nonflammable, it will support combustion. Its poor blood solubility permits rapid achievement of an alveolar and brain partial pressure of the drug. Analgesic effects of nitrous oxide are prominent, but skeletal muscle relaxation is minimal. Increasing awareness of the possible adverse effects related to the high-volume absorption of nitrous oxide and appreciation of potential toxic effects on organ function, may lead to a decline in the use of this anesthetic. Halothane Halothane is a halogenated alkane derivative that exists as a clear, nonflammable liquid at room temperature. The vapor of this liquid has a sweet nonpungent odor. An intermediate solubility in blood, combined with a high potency, permits rapid onset and recovery from anesthesia using halothane alone or in combination with nitrous oxide or injected drugs such as opioids. Despite its chemical stability, halothane is susceptible to decomposition to hydrochloric acid, hydrobromic acid, chloride, bromide, and phosgene. For this reason, halothane is stored in amber-colored bottles, and thymol is added as a preservative to prevent spontaneous oxidative decomposition. Thymol that remains in vaporizers following vaporization of halothane can cause vaporizer turnstiles or temperature-compensating devices to malfunction. Enflurane Enflurane is a halogenated methyl ethyl ether that exists as a clear, nonflammable volatile liquid at room temperature and has a pungent, ethereal odor. Its intermediate solubility in blood combined with a high potency permits rapid onset and recovery from anesthesia using enflurane alone or in combination with nitrous oxide or injected drugs such as opioids. Isoflurane Isoflurane is a halogenated methyl ethyl ether that exists as a clear, nonflammable, volatile liquid at room temperature and has a pungent, ethereal odor. Its intermediate solubility in blood combined with a high potency permits rapid onset and recovery from anesthesia using isoflurane alone or in combination with nitrous oxide or injected drugs such as opioids. Isoflurane is characterized by extreme physical stability, undergoing no detectable deterioration during 5 years of storage or on exposure to soda lime or sunlight. The stability of isoflurane negates the need to add preservatives such as thymol to the commercial preparation. Desflurane Desflurane is a fluorinated methyl ethyl ether that differs from isoflurane only by substitution of a fluorine atom for the chlorine found on the alpha-ethyl component of isoflurane. Solubility characteristics (blood:gas partition coefficient 0.42 and oil:gas partition coefficient 18.7) and potency (MAC 4.58%) permit rapid achievement of an alveolar partial pressure necessary for anesthesia followed by prompt awakening when desflurane is discontinued. The vapor pressure of desflurane at 20 C is 669 mmHg. The ratio of the fatal anesthetic concentration to that preventing movement to a painful stimulation (MAC) for desflurane in animals is 2.45 compared with 3.02 for isoflurane. Desflurane produces dose-related decreases in blood pressure and cardiac output that range from similar to somewhat greater than those evoked by equivalent doses of isoflurane. Infusions of epinephrine that provoke cardiac dysrhythmias during administration of desflurane and isoflurane are similar. Metabolism of desflurane is less than isoflurane, and the likelihood of organ damage from toxic metabolites seems remote. Indeed, plasma fluoride concentrations and renal or hepatic function tests do not change following inhalation of desflurane for about 90 minutes. Sevoflurane Sevoflurane is a fluorinated methyl ethyl ether with a vapor pressure of 170 mmHg at 20 C. Solubility characteristics (blood:gas partition coefficient 0.59 and oil:gas partition coefficient 55) and potency (MAC 1.71) permit rapid achievement of an alveolar partial pressure necessary for anesthesia followed by a prompt awakening when sevoflurane is discontinued. Inhalation of sevoflurane is not irritating to the airways, and there is a high degree of patient acceptance. Cardiovascular effects of sevoflurane appear to be similar to those of other volatile anesthetics. Defluorination of sevoflurane manifests as plasma fluoride concentrations that average 22 uM L -1 after 1 hour of administration. This peak level declines rapidly, often to near normal values in less than 1 hour, reflecting the poor overall lipid solubility of this drug. Table 1: physical & chemical properties of inhalational agents Molecular Weight (g) N2O 44.4 Halothane 197.4 Enflurane 184.5 Isoflurane 184.5 Desflurane 168 Sevoflurane 200 *Supports combustion. Boiling Point (C) -88 50.2 56.5 48.5 23.5 58.5 Vapor Pressure (mm Hg, 20C) 244 172 240 669 170 Chemical Stabilizer Yes No No No No Flammability Limits No* No No No 20.8% in O2 11% in O2 PHARMACOKINETICS OF INHALED ANESTHETICS: Pharmacokinetics of inhaled anesthetics describes their (1) absorption (uptake) from alveoli into pulmonary capillary blood, (2) distribution in the body, (3) metabolism, and (4) elimination, principally via the lungs. A series of partial pressure gradients beginning at the anesthetic machine serve to propel the inhaled anesthetic across various barriers (alveoli, capillaries, cell membranes) to their sites of action in the central nervous system. The principal objective of inhalation anesthesia is to achieve a constant and optimal brain partial pressure of the inhaled anesthetic. The brain and all other tissues equilibrate with the partial pressures of inhaled anesthetics delivered to them by arterial blood (Pa). Likewise, arterial blood equilibrates with the alveolar partial pressures (PA) of anesthetics. This emphasizes that the PA of inhaled anesthetics mirrors the brain partial pressure (Pbr). This is the reason that PA is used as an index of (1) depth of anesthesia, (2) recovery from anesthesia, and (3) anesthetic equal potency (MAC). It is important to recognize that equilibration between two phases means the same partial pressure exists in both phases. Equilibration does not mean equality of concentrations in two phases. Understanding those factors that determine the PA and thus the Pbr permits control of the doses of inhaled anesthetics delivered to the brain so as to maintain a constant and optimal depth of anesthesia. 1. 2. Induction of anesthesia occurs when an anesthetizing partial pressure has been achieved in the brain (Pbr). The brain can be considered as the final site for a series of concentration gradients in anesthetic partial pressures that begins with the concentration of anesthetic delivered from the anesthesia machine (Table 15- 2). The goal of inhalation anesthesia is to maintain an optimal and unchanging Pbr as reflected by the alveolar partial pressure (PA). The ability to clinically measure the Pa in the exhaled gases from the patient’s lungs permits the anesthesiologist to control the depth of anesthesia (Pbr). 3. The rate of induction of anesthesia is determined by the rate of rise of the Pa. During induction of anesthesia, blood returning to the lungs from tissues has a lower partial pressure (tissue uptake) than that in the alveoli. As a result, uptake of anesthetic occurs from alveoli and creates an inspired-toalveolar partial pressure difference. 4. Solubility (partition coefficients) of anesthetics in blood and tissues determines the time necessary for equilibration between two phases to occur (Table 15-3). Blood:gas solubility determines uptake from the alveoli into the blood and thus the rate of induction of anesthesia. Brain:blood solubility determines the time necessary for equilibration of partial pressures between the blood and brain. Concentration effect states that the higher the inspired partial pressure (Pi) the more rapid the increase in Pa. Second gas effect states that administration of high concentrations of N2O accelerates the rate of uptake of concomitantly inhaled gases (isoflurane, oxygen). 5. 6. 7. Recovery from anesthesia reflects reversal of the concentration gradients established during induction of anesthesia. In contrast to induction of anesthesia, the rate of recovery from anesthesia may be influenced by the duration of prior administration and the metabolism of the inhaled anesthetic. Recovery is most rapid following the short-duration administration of inhaled anesthetics that are poorly soluble in blood and tissues. Minimum Alveolar Concentration (MAC) Potency is commonly expressed as the MAC of the inhalational anesthetic. This is the alveolar concentration of anesthetic at 1 atmosphere (atm) that prevents movement in 50% of subjects in response to a painful stimulus. Various noxious stimuli have been used to provoke the response, including skin incision or electrical current. MAC reflects Pbr because the PA is in equilibrium with the brain. Clinically, it is necessary to administer 1.2–1.3 times MAC to prevent movement in at least 95% of patients. Combinations of inhaled anesthetics have additive effects on MAC (as a guide, 1% N2O usually decreases MAC for the volatile anesthetic by about 1% [may be less in children and with poorly soluble volatile anesthetics]). Comparison of effects of inhaled anesthetics on various organ systems is based on evaluation of equally potent MAC values for each drug (Table 2). The relative effects for anesthetics can be defined by calculating the anesthetic or therapeutic index, which is the dose producing a given effect divided by the MAC. For example, the dose of an anesthetic producing apnea when divided by the MAC defines its respiratory anesthetic index; an indication of the anesthetic’s margin of safety with respect to breathing. The MAC has also been useful in quantifying the effect of other drugs or pathophysiologic states on anesthetic requirement. TABLE 2. Anesthetic Requirements (MAC) for Inhaled Anesthetics MAC MAC (30 to 60 years (% with 60% old, 37C PB760,%) to 70% N2O) N2O 104 Halothane 0.74 0.29 Enflurane 1.68 0.60 Isoflurane 1.15 0.50 Desflurane 6.3 2.83 Sevoflurane 2.0 0.66 Factors That Influence MAC: Decrease MAC o Increasing age o Hypothermia o Other CNS depressants (opioids, benzodiazepines) o Decreased CNS concentrations of neurotransmitters (antihypertensives) o Acute alcohol ingestion o Alpha-2 agonists (clonidine) o Pregnancy Increase MAC o Hyperthermia o Chronic alcohol abuse (?) o Increased CNS concentrations of neurotransmitters (monoamine oxidase inhibitors) No Change in MAC o Gender o Anesthetic metabolism o Paco2 21 to 95 mm Hg EFFECTS OF INHALED ANESTHETICS ON ORGANS AND SYSTEMS A. 1. 2. 3. 4. 5. Central Nervous System Volatile anesthetics produce drug-specific and dose-dependent increases in cerebral blood flow (CBF) by virtue of their cerebral vasodilating effects (halothane > enflurane > isoflurane = desflurane = sevoflurane). N2O is a cerebral vasodilator but, because of its limited potency, it is associated with only modest increases in CBF. Increases in CBF produced by volatile anesthetics tend to normalize with time (CBF normalizes after 2 hours of halothane administration). Volatile anesthetics decrease cerebral metabolic oxygen requirements (CMRo2), with the largest decrease produced by isoflurane (flat electroencephalogram [EEG] at about 2 MAC). The CBF at which EEG evidence of ischemia occurs is lower with isoflurane than with halothane suggesting a possible cerebral protecting effect of isoflurane. Isoflurane is associated with better maintenance of the relationship between CMRO2 requirements and CBF, perhaps explaining the lower increase in CBF produced by this drug (theoretically the administration of isoflurane in the presence of pre-existing suppression of CMRO2 could result in exaggerated increases in CBF). In patients with decreased intracranial compliance, drug-induced increases in CBF produce parallel increases in cerebral blood volume and intracranial pressure (ICP). Volatile anesthetics can alter production and reabsorption of cerebrospinal fluid but, as with CBF, these effects normalize with time. In the presence of modest hypocapnia, isoflurane (probably also desflurane) appears less likely to produce potentially undesirable increases in ICP in patients with decreased intracranial compliance than enflurane or halothane. Enflurane is unique in producing dose-dependent spike-wave activity on the EEG, which is exaggerated by hypocapnia. Volatile anesthetics and N2O produce decreased amplitude and increased latency in the cortical components of somatosensory evoked potentials. B. Respiratory System 1. Ventilatory volumes and frequency of breathing. Volatile anesthetics produce drug-specific and dose-dependent depression of ventilation, as evidenced by increases in PaCO2 Decreases in tidal volume are incompletely offset by increases in rate of breathing (rapid, shallow breathing characteristic of the general anesthetic state) such that alveolar ventilation is decreased. 2. 3. 4. 5. C. Substitution of N2O for a portion of the volatile anesthetic results in less of an increase in the Paco2 at the same total MAC as with the volatile anesthetic alone. Effects of the intercostal muscles and diaphragm. Loss of intercostal muscle function with increasing doses of volatile anesthetics results in the characteristic rocking boat appearance of ventilation (chest collapses and abdomen protrudes as the diaphragm descends during inspiration) during general anesthesia. Chemical control of breathing Volatile anesthetics produce dose-dependent decreases in the ventilatory response to carbon dioxide, whereas even subanesthetic concentrations (0.1 MAC) of these drugs block the ventilatory response to hypoxemia. The absence of hyperpnea during arterial hypoxemia means that a useful clinical sign of hypoxia cannot be relied on during anesthesia. Assisted ventilation of the lungs to offset anesthetic-induced increases in Paco2 is of limited value because apnea occurs when Paco2 is lowered by about 5 mm Hg (apneic threshold). Surgical stimulation increases ventilation so as to decrease Paco2 by about 5 mm Hg. Airway caliber Volatile anesthetics are equally effective in decreasing airway resistance by causing bronchodilatation. Volatile anesthetics are equally effective for patients with asthma, although halothane and sevoflurane may be preferable to the other volatile anesthetics that have a more pungent odor that can cause airway irritation. Hypoxic pulmonary vasoconstriction. Volatile anesthetics in doses administered clinically do not seem to interfere with diversion of blood flow away from poorly ventilated or unventilated alveoli. Circulatory System 1. Comparative Circulatory Effects Decreased blood pressure D=H=I=E=S Increased heart rate D=E=I>H=S Decreased cardiac output H=E>D=I=S Decreased systemic vascular resistance S=D=I>E>H Cardiac sensitization H>D=I=E=S (H = halothane; E = enflurane; I = isoflurane; D = desflurane; S = sevoflurane.) o Increased heart rate during administration of isoflurane (also desflurane but at a higher dose) may reflect maintenance of baroreceptor activity in response to decreases in blood pressure. Minimal to absent changes in heart rate during administration of halothane- and sevoflurane- induced decreases in blood pressure suggest impairment of baroreceptor activity. o Abrupt increases in the alveolar concentration of desflurane and isoflurane (but not sevoflurane), produce transient increases in blood pressure and heart rate (magnitude less with isoflurane), which may reflect stimulation of irritant receptors in the airway leading to o o o 2. o o o o 3. o o increased sympathetic nervous system activity (blocked by prior administration of fentanyl or esmolol) Distribution of cardiac output is altered by anesthetics, with increased flow to the brain (halothane), skeletal muscles (isoflurane), and skin and decreased blood flow to the kidneys, liver, and gastrointestinal tract. Substitution of N2O for a portion of the volatile anesthetic results in less decrease in blood pressure at the same total MAC as with the volatile anesthetic alone. N2O produces a mild sympathomimetic effect manifesting as increases in systemic vascular resistance. When administered in the presence of opioids, N2O can decrease blood pressure and cardiac output. Cardiac dysrhythmias, conduction, and drug interactions Volatile anesthetics with an ether linkage are less likely than halothane to produce cardiac dysrhythmias in the presence of exogenous epinephrine injection. Children are less likely than adults to develop epinephrine-induced cardiac dysrhythmias. Volatile anesthetics exert a direct depressant effect on the sinoatrial node. Conduction of cardiac impulses is preserved through normal pathways better by isoflurane than by halothane. Myocardial depression produced by volatile anesthetics may be enhanced by calcium entry blockers and b antagonists. Coronary circulation Isoflurane, but not the other volatile anesthetics, may uncouple the generally close relationship between coronary blood flow and myocardial oxygen requirements. Isoflurane-induced dilation of intramyocardial arterioles, particularly in the presence of decreased coronary perfusion pressure and critical anatomic location of coronary artery stenosis, could divert blood flow away from areas of myocardium supplied by pressure dependent collateral blood vessels (coronary artery “steal”). D. Renal effects of volatile anesthetics are mainly a reflection of changes in blood flow to the kidneys. Anesthesia is typically associated with decreases in renal blood flow, glomerular filtration rate, and urine output. E. Volatile anesthetics have inherent skeletal muscle relaxant properties and potentiate the effects of nondepolarizing muscle relaxants. The mechanism of potentiation may involve desensitization of the postjunctional membrane or changes in skeletal muscle blood flow. Potentiation of nondepolarizing muscle relaxants is greatest with isoflurane, enflurane, desflurane, and sevoflurane, intermediate with halothane, and least with N2O. F. Metabolism and Toxicity Organ toxicity produced by volatile anesthetics reflects the production of metabolites and not the effects of the intact anesthetic molecule. Metabolism is largely dependent on tissue solubility of the anesthetic (determines reservoir available for exposure to the liver after the drug is discontinued) and the vulnerability of the drug to metabolism: Methoxyflurane 4% to 50% Halothane Sevoflurane Enflurane Isoflurane Desflurane 15% to 20% 3% 2.4% 0.2% 0.02% o Desflurane is the most resistant of the volatile anesthetics to metabolism. o Sevoflurane is metabolized to fluoride (nephrotoxicity does not appear to be a clinical problem) and degraded by soda lime and baralyme in a temperature dependent manner to an olefin (compound A, which in high concentrations in animals may be nephrotoxic and hepatotoxic) (Fig. 2). Fig. 2: rate of degradation by soda lime in relation to temperature. Sevoflurane is the least & desflurane is the most resistant to degradation. o In genetically susceptible patients, an oxidative trifluoroacetyl metabolite of halothane (also a potential metabolite of other fluorinated volatile anesthetics) may evoke the production of neoantigens directed against hepatocytes. Hematopoiesis o Prolonged exposure to N2O may produce anemia similar to pernicious anemia because of anesthetic-induced inhibition of methionine synthetase, which is involved in the metabolism of vitamin B12. o Volatile anesthetics, even with prolonged administration, do not appear to alter hematopoiesis. G. Surgical stress and endocrine response reflects the effects of the anesthetic and surgery (most important) and can be quantitated by measurement of plasma concentrations of cortisol and catecholamines. H. Uterine relaxation accompanies administration of all volatile anesthetics and can contribute to uterine blood loss when gravid patients are anesthetized. Inhaled drugs delivered to the parturient also cross the placenta and similarly affect the fetus.