ACID
advertisement
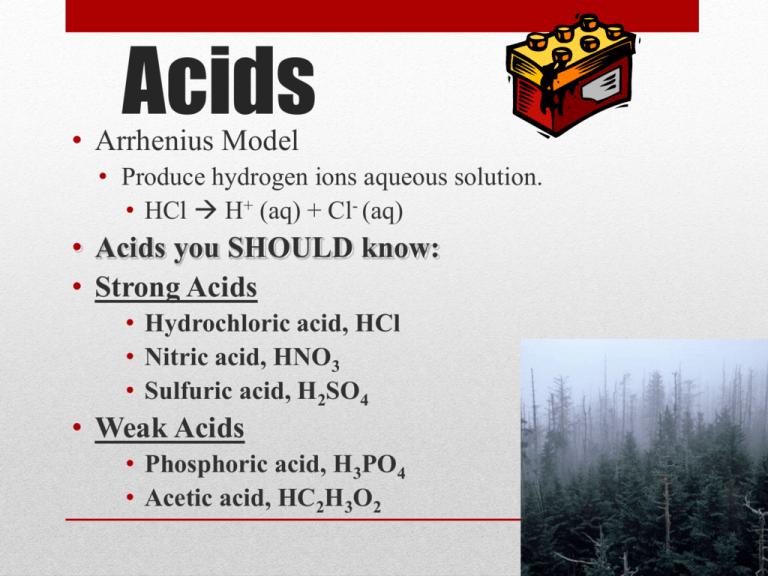
Acids • Arrhenius Model • Produce hydrogen ions aqueous solution. • HCl H+ (aq) + Cl- (aq) • Acids you SHOULD know: • Strong Acids • Hydrochloric acid, HCl • Nitric acid, HNO3 • Sulfuric acid, H2SO4 • Weak Acids • Phosphoric acid, H3PO4 • Acetic acid, HC2H3O2 Properties of Acids • Acids taste sour • Acids affect indicators • Blue litmus turns red • • • • • • Methyl orange turns red Acids have a pH lower than 7 Acids are proton (hydrogen ion, H+) donors Acids react with active metals, produce H2 Acids react with carbonates Acids neutralize bases Bases • Arrhenius Model • Produce Hydroxide ions. • NaOH Na+ (aq) + OH- (aq) • Examples of Bases • • • • Sodium hydroxide (lye), NaOH Potassium hydroxide, KOH Magnesium hydroxide, Mg(OH)2 Calcium hydroxide (lime), Ca(OH)2 Properties of Bases • Bases taste bitter • Bases affect indicators • Red litmus turns blue • Phenolphthalein turns purple • Bases have a pH greater than 7 • Bases are proton (hydrogen ion, H+) acceptors • Solutions of bases feel slippery • Bases neutralize acids Bronsted – Lowery Model • ACID: Substance that can donate proton (H+1). • BASE: Substance that can accept proton (must contain lone pair of electrons). The pH Scale • • • • The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic. The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value. • For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. • The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. Calculating pH or pOH • pH = -log[H+] • Example [H+] = 1.0 x 10-9 • Answer pH=9.0 • pOH = -log[OH+] • Example [OH+] = 1.0 x 10-6 • Answer pOH = 6 • pOH + pH = 14.0 Acids Neutralize Bases • HCl + NaOH NaCl + H2O • Neutralization reactions ALWAYS produce a salt and water. • H2SO4 + 2NaOH Na2SO4 + 2H2O • 2HNO3 + Mg(OH)2 Mg(NO3)2 + 2H2O Bases Neutralize Acids Milk of Magnesia contains magnesium hydroxide, Mg(OH)2, which neutralizes stomach acid, HCl. 2 HCl + Mg(OH)2 MgCl2 + 2 H2O Titration: • A technique for determining the concentration of a solution. 12 Acid Base Titration • Titration is a method for determining the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. • In a titration procedure, a measured volume of an acid or base of unknown concentration is placed in a beaker or flask, and initial pH recorded. • A neutralization reaction is a reaction in which an acid and a base in an aqueous solution react to produce a salt and water. • A salt is an ionic compound made up of a cation from a base and an anion from an acid. • Neutralization is a double-replacement reaction. Titration Procedure 1) A measured volume of an acidic or basic solution of unknown concentration is placed in a beaker. The electrodes of a pH meter are immersed in this solution, and the initial pH of the solution is read and recorded. 2) A buret is filled with the titrating solution of known concentration. This is called the standard solution, or titrant. 3) Measured volumes of the standard solution are added slowly and mixed into the solution in the beaker. The pH is read and recorded after each addition. This process continues until the reaction reaches the equivalence point, which is the point at which moles of H+ ion from the acid equal moles of OH- ion from the base. 16 • Standard Solution or titrant– An acid/ base solution whose concentration is known. • End point: The point at which an indicator changes color. • Equivalence point: The point at which moles of H+ ion from the acid equals moles of OH– ion from the base. An abrupt change in pH occurs at the equivalence point. Acid-Base Indicators • Chemists often use a chemical dye rather than a pH meter to detect the equivalence point of an acid-base titration. • Chemical dyes whose colors are affected by acidic and basic solutions are called acid-base indicators. • Many natural substances act as indicators. – If you use lemon juice in your tea, you might have noticed that the brown color of tea gets lighter when lemon juice is added. – Tea contains compounds called polyphenols that have slightly ionizable hydrogen atoms and therefore are weak acids. – Adding acid in the form of lemon juice to a cup of tea lessens the degree of ionization, and the color of the un-ionized polyphenols becomes more apparent. • Chemists have several choices in selecting indicators. – Bromthymol blue is a good choice for the titration of a strong acid with a strong base, and phenolphthalein changes color at the equivalence point of a titration of a weak acid with a strong base. 19 • Chemical dyes whose color are affected by acidic and basic solutions are called acid-base indicators. Indicators and Titration End Point • Many indicators used for titrations are weak acids. – Each has its own particular pH or pH ranges over which it changes color. • The point at which the indicator used in a titration changes color is called the end point of the titration. – It is important to choose an indicator for a titration that will change color at the equivalence point of the titration. – Remember that the role of the indicator is to indicate to you, by means of a color change, that just enough of the titrating solution has been added to neutralize the unknown solution. • Equivalence point ≠ End point! – BUT for strong-strong titrations, the pH change is so steep and so large, that the are approximately equal. 21 Titration with an Indicator 22







