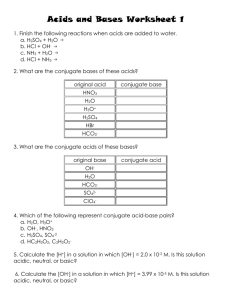
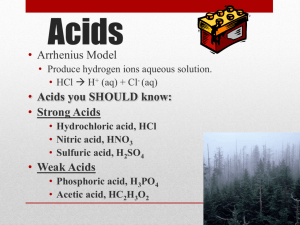
Acid-Base Test Review Guide
advertisement
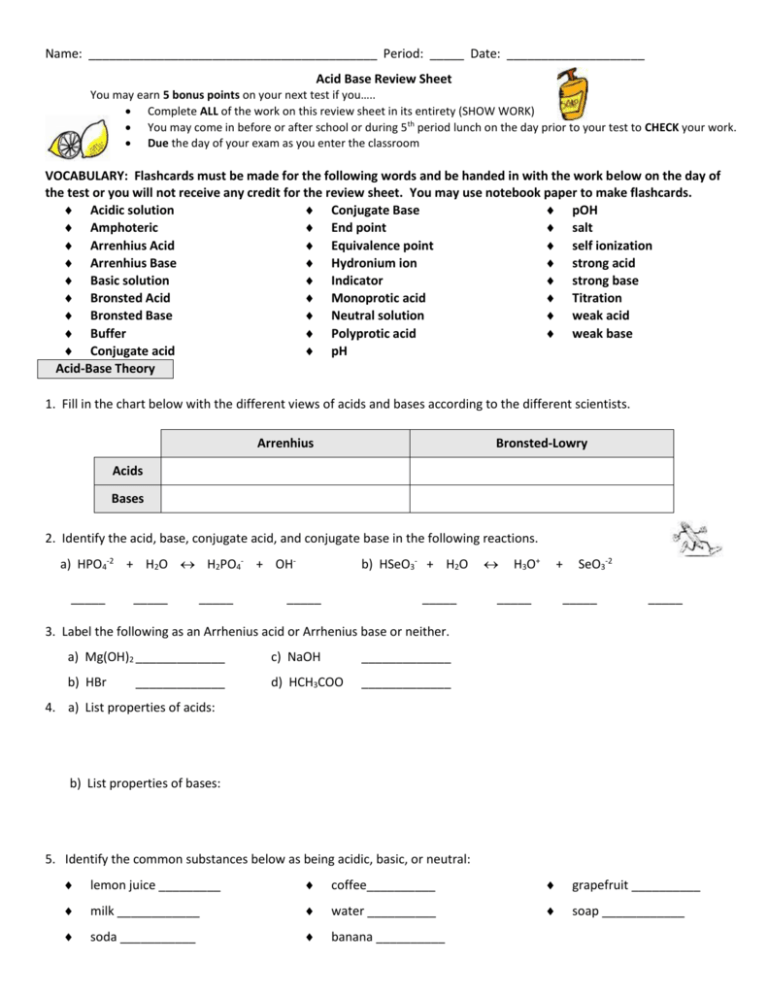
Name: __________________________________________ Period: _____ Date: ____________________ Acid Base Review Sheet You may earn 5 bonus points on your next test if you….. Complete ALL of the work on this review sheet in its entirety (SHOW WORK) You may come in before or after school or during 5th period lunch on the day prior to your test to CHECK your work. Due the day of your exam as you enter the classroom VOCABULARY: Flashcards must be made for the following words and be handed in with the work below on the day of the test or you will not receive any credit for the review sheet. You may use notebook paper to make flashcards. Acidic solution Conjugate Base pOH Amphoteric End point salt Arrenhius Acid Equivalence point self ionization Arrenhius Base Hydronium ion strong acid Basic solution Indicator strong base Bronsted Acid Monoprotic acid Titration Bronsted Base Neutral solution weak acid Buffer Polyprotic acid weak base Conjugate acid pH Acid-Base Theory 1. Fill in the chart below with the different views of acids and bases according to the different scientists. Arrenhius Bronsted-Lowry Acids Bases 2. Identify the acid, base, conjugate acid, and conjugate base in the following reactions. a) HPO4-2 + H2O H2PO4- + OH_____ _____ _____ b) HSeO3- + H2O _____ _____ H3O+ + _____ SeO3-2 _____ _____ 3. Label the following as an Arrhenius acid or Arrhenius base or neither. a) Mg(OH)2 _____________ c) NaOH _____________ b) HBr d) HCH3COO _____________ _____________ 4. a) List properties of acids: b) List properties of bases: 5. Identify the common substances below as being acidic, basic, or neutral: lemon juice _________ coffee__________ grapefruit __________ milk ____________ water __________ soap ____________ soda ___________ banana __________ 6. Identify the following acids as monoprotic or polyprotic. a) H2CO3 __________________________ c) HCl ______________________ b) H3PO4 __________________________ d) H2SO3 ______________________ 6. Using the diagram below, explain why water is considered to be amphoteric. Acid-Base Properties 10. What is an indicator? 11. List three types of indicators that we have learned about: a) ______________________ b) ______________________ c) ______________________ 14. With which of the following will HNO3 react? Circle all that apply. a) KOH c) Zn b) CaCO3 d) Cu 15. With which of the following would NaOH react? Circle all that apply. a) HCN c) CaCO3 b) KOH d) H2S 16. Examine the data in the table. Fill in the blank spaces with the appropriate term. Substance Red Litmus Blue Litmus Reaction with Phenolphthalein stays red turns red stays clear Unknown A Unknown B Unknown C Acid, Base, 0r Neutral? neutral turns blue stays blue turns pink a) Which of the unknowns above would have more hydrogen ions? ___________________ b) Which of the unknowns above would have a pH above 7? ___________________ pH and pOH 17. a) [H+][OH-]=________ b) What is the equation for pH? ___________________ c) What is the equation for pOH? _________________ d) _____ + _____ = 14 18. a) [H+] = [OH-] describes a substance that is ____________. b) [H+] > [OH-] describes a substance that is ____________. c) [H+] < [OH-] describes a substance that is ____________. 19. Fill in the blanks in the following chart, and then answer the questions below. SHOW YOUR WORK. Substance Acidic, Alkaline, or Neutral? Unknown A Unknown B [H+] [OH-] pH pOH 6 2.50 x 10-8 M Unknown C 4 Unknown D 1.3 x 10-10 M 1.0 x 10-7 M Unknown E a) Which substance is the most acidic? b) Which substance is the most basic? ______________ _______________ Strong vs. Weak Acids and Bases 20. a) What determines the strength of an acid or base? _________________________________________ b) Is it correct to state that a strong acid is a concentrated acid and that a weak acid is a dilute acid? Explain. 21. Acid A and acid B are tested with a conductivity apparatus. When the conductivity apparatus is placed in acid A, the bulb glows dimly. When it is placed in acid B, the bulb glows more brightly. Which acid is stronger, and how do you know? 22. List the 6 strong acids. _____________ 23. What elements make the strongest bases? _______________________________ Acid Nomenclature and Formula Writing 24. Fill in the blanks in the following chart by either giving the acid formula or acid name. Acid Formula a) Acid Name HNO2 b) Hydrosulfuric Acid c) HClO3 d) HI e) Sulfuric Acid Neutralization Reactions 25. When an acid reacts with a base a ________________________________ reaction takes place. This means ________________ and __________________ are produced. 26. Explain how a salt is formed. 27. Write and balance each of the following neutralization reactions. a) hydroiodic acid and ammonium hydroxide b) phosphorous acid and calcium hydroxide c) hydrocyanic acid and lithium hydroxide d) Which acid and base would form cesium selenide and water? Titrations 28. The first thing you should do for a titration problem is to write a __________________ equation. A titration can be used to find the unknown ____________________of an acid. 29. For every titration an ____________________ must be used in order to see when the ___________________ is reached. 30. In a titration, the point at which the amount of moles of acid is equal to the amount of moles of base is called the ________________________________________. Calculations: 31. What is the concentration (molarity) of sodium hydroxide if 20.0 mL of the solution is neutralized by 28.0 mL of 1.00 M hydrochloric acid? 32. In lab, a titration is performed. 50.0 mL of hydrosulfuric acid reacts with 34.9 mL of 3.89 M sodium hydroxide. What is the concentration (molarity) of the hydrosulfuric acid solution? 33. A student titrates a 20.00 mL sample of a solution of HBr with unknown molarity. The titration requires 20.05 mL of a 0.1819 M solution of NaOH. What is the molariy of the HBr solution?