Worksheet - Acids and Bases
advertisement

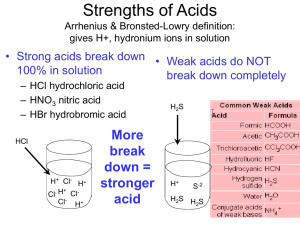
Name Class Date Acids and Bases 1. Compare properties of acids and bases. Circle any properties that they have in common. ACIDS BASES Taste Feel Reaction/Metals Electrolyte Indicator – Litmus Indicator-Phenolphthalein How is it related to H+? 2. What is the formula for the hydronium ion? 3. How does a chemist define each of the following terms? a. Weak b. Strong c. Dilute d. Concentrated 4. Give an example of each of the following. a. A weak acid b. A weak base c. A strong acid d. A strong base 5. What is neutralization? 6. What are the products if equal amounts of an acid and a base react? 7. Is acid/base neutralization endothermic or exothermic? 8. Define and give two examples of polyprotic acids. 9. What is a titration? 10. What laboratory glassware is used in a titration? 11. What is true about the amount of H+ (acid) and OH- (base) in a solution at the equivalence point of a titration? 12. How can we know that the endpoint in a titration has been reached? 13. In an acid/base neutralization, the cation of the base and the anion of the acid combine to make a salt. What acid and base must have combined to make each of the following salts? NaCl NH4Cl Na2SO4 K3PO4 KNO3 Ca(NO3)2 14. At the equivalence point, moles of acid in the solution = moles of base. For monoprotic acids and bases, we can express this mathematically as MaVa = MbVb a. 38.0 mL of 0.10M HCl is needed to neutralize 25.0 mL to NH4OH. What is the molarity of the ammonium hydroxide? b. 29.5mL of 0.15M NaOH neutralizes 25.0mL of HNO3. What is the molarity of the acid? c. 30.0mL of 0.20M HCl will be neutralized with 0.50M NaOH. What volume of the base is needed?