E. coli S. aureus - York College of Pennsylvania
advertisement
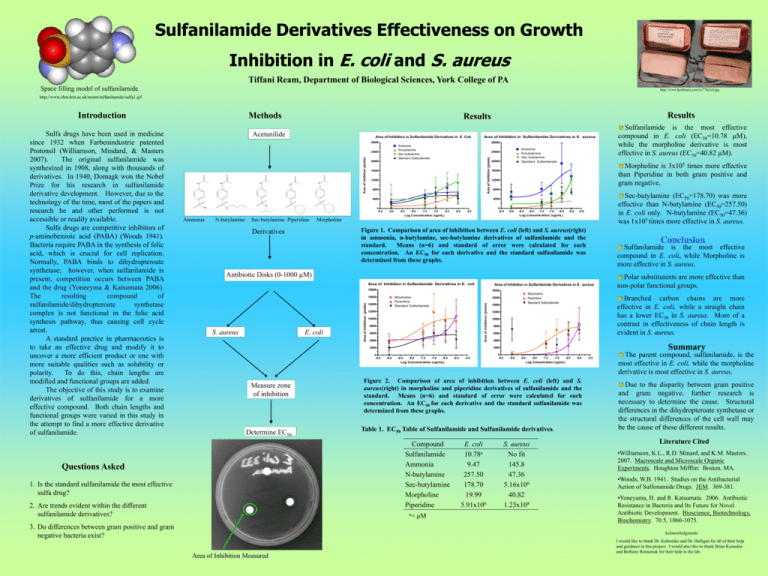
Sulfanilamide Derivatives Effectiveness on Growth Inhibition in E. coli and S. aureus Tiffani Ream, Department of Biological Sciences, York College of PA Space filling model of sulfanilamide. http://www.ljmilitaria.com/1c77fe2c0.jpg http://www.chm.bris.ac.uk/motm/sulfanilamide/sulfa1.gif Introduction Results Results Acetanilide Area of Inhibition in Sulfanilamide Derivatives in E. Coli Area of Inhibition in Sulfanilamide Derivatives in S. aureus 28000 28000 Ammonia N-butylamine Sec-butylamine Standard Sulfanilamide 20000 16000 12000 8000 20000 -9.0 -8.5 -8.0 -7.5 -7.0 -6.5 -6.0 16000 12000 8000 0 -9.5 -5.5 -9.0 N-butylamine Sec-butylamine Piperidine Sec-butylamine (EC50=178.70) was more effective than N-butylamine (EC50=257.50) in E. coli only. N-butylamine (EC50=47.36) was 1x105 times more effective in S. aureus. -8.5 -8.0 -7.5 -7.0 -6.5 -6.0 -5.5 Log Concentration (ug/mL) Log Concentration (ug/mL) Ammonia Morpholine Figure 1. Comparison of area of inhibition between E. coli (left) and S. aureus(right) in ammonia, n-butylamine, sec-butylamine derivatives of sulfanilamide and the standard. Means (n=6) and standard of error were calculated for each concentration. An EC50 for each derivative and the standard sulfanilamide was determined from these graphs. Derivatives Conclusion Sulfanilamide is the most effective compound in E. coli, while Morpholine is more effective in S. aureus. Antibiotic Disks (0-1000 µM) Area of Inhibition in Sulfanilamide Derivatives in E. coli Area of Inhibition in Sulfanilamide Derivatives in S. aureus 18000 Morpholine Piperidine Standard Sulfanilamide 14000 12000 10000 8000 6000 4000 -9.0 -8.5 -8.0 -7.5 -7.0 -6.5 -6.0 -5.5 Determine EC50 1. Is the standard sulfanilamide the most effective sulfa drug? 2. Are trends evident within the different sulfanilamide derivatives? 8000 6000 4000 Summary 0 -9.5 -9.0 -8.5 -8.0 -7.5 Table 1. EC50 Table of Sulfanilamide and Sulfanilamide derivatives. a= 3. Do differences between gram positive and gram negative bacteria exist? 10000 -7.0 -6.5 -6.0 Figure 2. Comparison of area of inhibition between E. coli (left) and S. aureus(right) in morpholine and piperidine derivatives of sulfanilamide and the standard. Means (n=6) and standard of error were calculated for each concentration. An EC50 for each derivative and the standard sulfanilamide was determined from these graphs. Compound Sulfanilamide Ammonia N-butylamine Sec-butylamine Morpholine Piperidine Questions Asked 12000 Log Concentration (ug/mL) Log Concentration (ug/mL) Measure zone of inhibition 14000 Branched carbon chains are more effective in E. coli, while a straight chain has a lower EC50 in S. aureus. More of a contrast in effectiveness of chain length is evident in S. aureus. 2000 2000 0 -9.5 Morpholine Piperidine Standard Sulfanilamide 16000 Area of Inhibition (pixels) E. coli Polar substitutents are more effective than non-polar functional groups. 18000 16000 S. aureus Sulfanilamide is the most effective compound in E. coli (EC50=10.78 µM), while the morpholine derivative is most effective in S. aureus (EC50=40.82 µM). Morpholine is 3x105 times more effective than Piperidine in both gram positive and gram negative. 4000 4000 0 -9.5 Ammonia N-butylamine Sec-butylamine Standard Sulfanilamide 24000 Area of Inhibition (pixels) Area of Inhibition (pixels) 24000 Area of Inhibition (pixels) Sulfa drugs have been used in medicine since 1932 when Farbenindustrie patented Protonsil (Williamson, Mindard, & Masters 2007). The original sulfanilamide was synthesized in 1908, along with thousands of derivatives. In 1940, Domagk won the Nobel Prize for his research in sulfanilamide derivative development. However, due to the technology of the time, most of the papers and research he and other performed is not accessible or readily available. Sulfa drugs are competitive inhibitors of p-aminobenzoic acid (PABA) (Woods 1941). Bacteria require PABA in the synthesis of folic acid, which is crucial for cell replication. Normally, PABA binds to dihydropteroate synthetase; however, when sulfanilamide is present, competition occurs between PABA and the drug (Yoneeyma & Katsumata 2006). The resulting compound of sulfanilamide/dihydropteroate synthetase complex is not functional in the folic acid synthesis pathway, thus causing cell cycle arrest. A standard practice in pharmaceutics is to take an effective drug and modify it to uncover a more efficient product or one with more suitable qualities such as solubility or polarity. To do this, chain lengths are modified and functional groups are added. The objective of this study is to examine derivatives of sulfanilamide for a more effective compound. Both chain lengths and functional groups were varied in this study in the attempt to find a more effective derivative of sulfanilamide. Methods µM E. coli 10.78a 9.47 257.50 178.70 19.99 5.91x106 S. aureus No fit 145.8 47.36 5.16x106 40.82 1.23x108 -5.5 The parent compound, sulfanilamide, is the most effective in E. coli, while the morpholine derivative is most effective in S. aureus. Due to the disparity between gram positive and gram negative, further research is necessary to determine the cause. Structural differences in the dihydropteroate synthetase or the structural differences of the cell wall may be the cause of these different results. Literature Cited •Williamson, K.L., R.D. Minard, and K.M. Masters. 2007. Macroscale and Microscale Organic Experiments. Houghton Mifflin: Boston, MA. •Woods, W.B. 1941. Studies on the Antibacterial Action of Sulfonamide Drugs. JEM. 369-381. •Yoneyama, H. and R. Katsumata. 2006. Antibiotic Resistance in Bacteria and Its Future for Novel Antibiotic Development. Bioscience, Biotechnology, Biochemistry. 70.5, 1060-1075. Acknowledgments Area of Inhibition Measured I would like to thank Dr. Kaltreider and Dr. Halligan for all of their help and guidance in this project. I would also like to thank Brian Kanaskie and Bethany Remeniuk for their help in the lab.