Sulfa Drug Group Project
advertisement

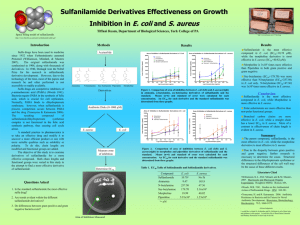
Sulfa Drug Group Project Chemistry 258 Loyd D. Bastin Brief History 1932-I.G. Farben (dye company in Germany) 1933-Prontosil used against staphylococcal septicemia (blood infection) 1935-Gerhard Domagk Prontosil cured streptococcal infections in mice and rabbits Later shown to have an even broader application 1939-Domagk earned Nobel Prize in medicine Hitler did not allow him to accept Was later awarded the prize More History 1935-J. Tréfouël In vivo (in animals) prontosil effective In vitro (petri dish) prontosil ineffective WHY? Tréfouël showed that prontosil was metabolized to sulfanilamide in the body Sulfanilamide shown to have in vivo and in vitro activity Led to an explosion in the synthesis of sulfanilamide analogs Became the wonder drug of the 30s and early 40s (referred to as sulfa drugs) A Little More History 1929-Alexander Fleming discovers Penicillin G 1941-Penicillin G shown to be successful antibacterial agent in humans Leads to a decline in use of sulfa drugs However, sulfa drugs are still used for malaria, tuberculosis, leprosy, meningitus, pneumonia, scarlet fever, plague, respiratory, infections, and intestinal/urinary tract infections Synthesis Your Task Groups of 3 or 4 You are a team and it is your job to work together and synthesize the appropriate number of sulfa drugs. Choose the R groups and synthesize 3 (or 4) different sulfanilamide analogs. See handout for groups Weekly group meetings with me to discuss your progress future plans Group of 3 – synthesize 3 different sulfanilamide analogs Group of 4 – synthesize 4 different sulfanilamide analogs Design a “greener” experiment for one of your sulfanilamide analogs Your Task Continued Here are your possible R groups Group A (Should wor k well) Group B (harder) Group C (even Harder) Ammonia methyl benzy l ami ne 2-ami noace tophenone 2-aminothiazole isopropyl amine p-bromoanili ne butyl ami ne sec-butyl ami ne o-anisidine propyl ami ne phene thyl ami ne ethyl- 4-ami no benzoa te benzy l ami ne anili ne 2,4,6-tribromoanil ine p-tolu idine 2-methyl-4-nit roanili ne cyc lohexy l ami ne 2-methoxy-4 -nit roanili ne p-ami nopheno l It is your job to make the synthesis work. Use the procedures in the lab manual, Pavia, and/or Shriner as GUIDELINES. Modifications will be necessary. Could be as simple as changing T and/or solvent, or it could require some research on your part. Use your time wisely and multi-task. Your group must submit IN WRITING at your first group meeting, which molecules you plan to synthesize. If you have trouble with compounds from Groups B or C, you can update your target molecules after the first meeting. However, you can NOT upgrade your target group after the first group meeting. (example, you can not go from group A target to group B target) Your Task Continued If you change your plan, it must be submitted in writing and signed by all group members. Then you will test the antibacterial activity of your drugs against E. coli. The written lab report 52 points (total possible) Successful synthesis of drug from group A: 4 pts (0 points if not made) Successful synthesis of drug from group B: 7 points " Successful synthesis of drug from group C: 12 points " Examples: The total points possible for doing an amine from group A and 3 amines from group C would be 92 points (52 + 4 + 12 +12 + 12). Total possible for doing 4 amines from group A would be 68 points (52 + 4 + 4 +4 +4). You MUST prove the identity and purity of your compound to receive all of the points using mp, NMR, IR, and/or TLC We will do NMR once you have purified your product and shown this by TLC. Final Report Each group will submit ONE report. Each member will submit a grade sheet for each member of their group For details please see pg. 33 in lab manual and then ask me if you have any questions.