Crystallization
advertisement

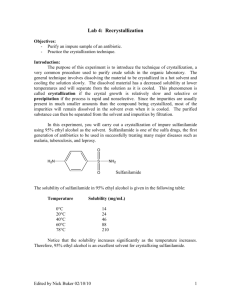
CHEM 12A—Trego Crystallization of Sulfanilamide and Fluorene Submitted by: Click here to enter text. Partner: Click here to enter text. Date Submitted: Click here to enter text. Abstract Click here to enter text. Part A Melting Point Data Impure Sulfanilamide Click here to enter text. Crystallized Sulfanilamide Click here to enter text. Based on the melting point of the crystallized sulfanilamide, was it pure? How do you know? (You should compare the experimental melting point to the literature value.) As well, comment on the color of the product and what it suggests about the purity of the crystallized sulfanilamide. Click here to enter text. Mass Data Mass of Impure Sulfanilamide Click here to enter text. Mass of Crystallized Sulfanilamide Percent Recovery Click here to enter text. Click here to enter text. Comment on the percent recovery and propose possible sources of product loss. Click here to enter text. Part C Describe the results of finding the best solvent for the crystallization of fluorene. Click here to enter text. Explain the results for finding the best solvent in terms of polarity and solubility. Click here to enter text. Melting Point Data Impure Fluorene Click here to enter text. Crystallized Fluorene Click here to enter text. Mass Data Impure Fluorene Click here to enter text. Crystallized Fluorene Click here to enter text. Percent Recovery Click here to enter text. Comment on the percent recovery and propose possible sources of product loss. Click here to enter text. The solubility of fluorene in each of the three solvents used in Part C corresponds to one of the three curves shown in figure 11.1 (Technique 11). Identify which curve in 11.1 best describes the solubility of fluorene in each of the three solvents. Click here to enter text. Questions Respond to questions 1-3 on page 33 of Pavia Question 1 Click here to enter text. Question 2 Click here to enter text. Question 3 Click here to enter text. Please attach your carbonless copy pages.