File - Amanda Zabotsky
advertisement

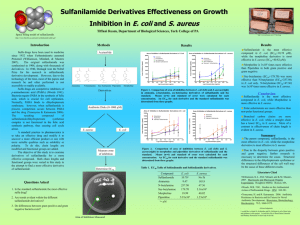
Amanda Zabotsky Section3 October 31, 2007 Dr. Zirpoli Crystallization of sulfanilamide. Introduction The purpose of this experiment was to crystallize a sample of sulfanilamide and create a more pure sample from a tainted sample and retrieve a relative melting point by the use of a melting point apparatus and compare the samples and determine if they were near the given melting points as found on www.chemfinder.com. Reagent Table Items included in this table were pulled from the chemfinder.com website. Molec. Molec. Melting formula weight pt Sulfanilamide C6H8N2O2S 172.201 164.5— Boiling pt density -- 1.08 166.5 C Ethyl alcohol C2H6O 46.068 -114.1 C 78.3 C 1.6 acetanilide C8H9NO 135.16 114.3 C 304.0 C -- Procedure: 9.5mL of ethyl alcohol was added to 1.50 g of impure sulfanilamide and was then placed in a 25 mL Erlenmeyer flask and brought to a boil on a hotplate and was dissolved within a few minutes. Once this was achieved it was placed in an ice bath with an air current blown over the lip of the Erlenmeyer flask. Once the crystals formed this was transferred to a vacuum filter using a Buchner funnel where the remaining liquid was pulled to the flask below and the crystals remained above. The crystals were then set aside to dry and placed in a vial until the following lab period where they were pulverized and placed in a melting point tube then placed in a melting point apparatus with a sample of impure sulfanilamide and the melting points were compared to those found on the chemfinder.com website. Results: Upon the crystallization of the Sulfanilamide the melting point of the pure sample was determined to have the melting point of 164- 166 C and the impure was found to melt at 151-152 C. the melting point of the purified sulfanilamide was in the range that was provided by chemfinder.com. The amount of recovery was found to be 67.087% Because of the equation of: (recovered pure/ initial impure) x 100. Or (1.011 / 1.507) x 100 = 67.087. This is found to be an adequate amount because crystallization is a wasteful experiment however still efficient. Some places where errors may have occurred is in the transferring between the 25 ml Erlenmeyer flask and the Buchner funnel some crystals may have been left over, some may have been lost from over—evaporation of the ethyl alcohol or to human error with the reading of the scale. Discussion: Although crystallization is wasteful it was a better option to retrieve the melting point of the sulfanilamide because it was combined with ethyl alcohol at two vastly different temperatures where if the use of a distillation, be it fractional, steam, or simple; the treatment at two different temperatures would not have had the same effects and had the result of having the suggested boiling point that was found on the chemfinder.com website. References: Pavia, D. L., Lampman, G. M., Kriz, G. S., & Engle, R. G. (2005). Introduiction to organic laboratory techniques a small scale approach. canada:Thomas Learning , INC. “Chemfinder.com” Chemfinder.com database and internet searching. 2004. Oct. 16, 2007 Http:// www.chemfinder.com