Carboxylic Acids and Nitriles
advertisement

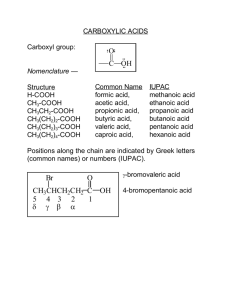
Carboxylic Acids and Nitriles Organic Chemisty 6e Chapter 20 Introduction to Chapter Chapter 20 Introduction RCO2H – About Carboxylic Acids – Serve as starting materials for preparing acyl derivaties; • Esters • Amides • Acid Chlorides – Many carboxylic acids are found in Nature • Acetic Acid, CH3CO2H for vinegar • Butanoic Acid, CH3(CH2)2CO2H is the rancid oder from sour butter • Palmitic Acid, CH3(CH2)14CO2H is a biological precursor of fats and other lipids. – Approx. 2 million tons of acetic acid are produced annually in the United States 20.1 Naming Carboxylic Acids and Nitriles Nomencalture - RCOOH Two systems have been adopted by IUPAC: 1. Carboxylic acids derived from open-chain alkanes are named by replacing their terminal –e of the alkane with -oic acid O O 3 H3 C H3C 5 OH 4 1 2 OH CH3 Propanoic acid 4-Methylpentanoic acid 2. Compounds that have a –COOH group bonded to a ring are named using the suffix –carboylic acid CO2H CO2H 1 1 6 2 5 3 4 5 Br 3-Bromocyclohexanecarboxylic acid 2 4 3 1-Cyclopentenecarboxylic acid 20.1 Naming Carboxylic Acids and Nitriles Nomencalture – RC N Two systems have been adopted by IUPAC: 1. Nitriles derived from open-chain alkanes are named by adding –nitrile as a suffix to the alkane name, with the nitrile carbon numbered C1: 2 3 H3C 5 4 CN 2 3 CN 1 1 4 H3 C 6 CH3 5 CH3 CH3 4-Methylpentanenitrile 4,5-dimethylhexanenitrile 2. Nitriles are named as derivatives of carboxylic acids by replacing the –ic acid or –oic acid with –onitrile, or replacing –carboxylic acid with -carbonitrile. CH3 2 NC H3C CH3 1 3 6 4 C N C 5 Acetonitrile 3,3-Dimethylcyclohexanecarbonitrile Benzonitrile N 20.1 Naming Carboxylic Acids and Nitriles 20.2 Structure and Physical Properties Carboxyl carbon has sp2 hybridization; carboxyl group is therefore planar with C C O and O C O bond angles of ~120° O H H O H H Carboxylic acids are strongly associated by hydrogen bonds, most existing as cyclic dimers held together by two hydrogen bods O H O H3 C CH3 O H O 20.2 Structure and Physical Properties O H O OH H3 C Formic acid OH Acetic acid O C OH O O H3 C H2 C OH Propanoic acid OH Propenoic acid Benzoic acid 20.3 Dissociation of Carboxylic Acids Carboxylic acids are acidic, Ka ~ 10-5 (pKa ~5), and therefore react readily with a base such as NaOH to give a carboxylate salt. O O + R NaOH + OH - R H2O + O Na Metal-carboxylate salt O O + R + H2O R OH - O [ RCO 2 ][ H 3 O ] Ka [ RCO 2 H ] and pKa = - log Ka H3O+ 20.3 Dissociation of Carboxylic Acids Relative Acidity of Carboxylic Acids Carboxylic acids are more acidic than alcohols by a factor of ~1011 O CH3CH2OH CH3COH HCl pKa = 16 pKa = 4.75 pKa = -7 Acidity Explanation: Acidity can be explained in terms of bonding H3 C OH + H2O - H3C O + H3O+ Not stabilized O R + OH - O H2O O R R O - stabilized by resonance O Key Points 20.4 Substituent Effects on Acidity Acidity of carboxylic acids varies greatly according to the nature of the substituent attached to the carboxyl group Generally, any factor that STABILIZES the carboxylate group relative to the undissociated acid will drive the equilibrium toward increased dissociation and result in increased acidity. O EWG C O - O Electron-withdrawing group Stabilizes carboxylate and strengthens acid EWG C - O Electron-donating group Destabilizes carboxylate and weakens acid Electron-withdrawing groups stabilize carboxylate ions Electron-donating groups destabilize carboxylate ions Relative Strengths of Acetic Acid and Chloro- Derivatives 20.4 Substituent Effects on Acidity O O H O Cl Cl OH H O Cl OH H OH Cl H Cl H pKa = 4.75 OH H pKa = 2.85 Cl pKa = 1.48 pKa = 0.64 Acidity O ClCH2CH2CH2COH pKa = 4.52 Cl O CH3CHCH2COH pKa = 4.05 Acidity Cl O CH3CH2CHCOH pKa = 2.86 Substituent Effects in Substiruted Benzoic Acids 20.5 Deactivating groups (electron-withdrawing) stabilize carboxylates Activating groups (electron-donating) destabilize carboxylates O O O C C C OH OH O2 N CH3O p-Methoxybenzoic acid (pKa = 4.46) OH Benzoic acid (pKa = 4.19) Acidity p-Nitrobenzoic acid (pKa = 3.41) Five methods of preparation of Carboxylic Acids 20.6 Preparation of Carboxylic Acids I - Oxidation of a substituted alkylbenzene using KMnO4 or Na2Cr2O7 O NO2 CH3 KMnO4 H2O, 95°C p-Nitrotoluene NO2 C OH p-Nitrobenzoic acid (88%) * Oxidation occurs for 1° & 2° alkyl groups only, not in 3° alkyl groups II – Oxidative cleavage of an alkene with KMnO4 O CH3(CH2)7CH CH(CH2)7COH Oleic acid O KMnO4 H3O+ O O CH3(CH2)7COH + HOC(CH2)7COH nonanoic acid * Alkene must have at least one vinylic hydrogen nonanedioic acid III – Oxidation of a 1° alcohol or an aldehyde O 20.6 Preparation of Carboxylic Acids CH3(CH2)8CH2COH 1-Decanol CrO3 H3O+ CH3(CH2)8COH Decanoic acid (93%) O CH3(CH2)4CH Hexanal O Ag2O NH4OH CH3(CH2)4COH Hexanoic acid (85%) * 1° alcohols are oxidized with CrO3 in aqueous acid, aldehydes are oxidized with either acidic CrO3 OR Tollen’s reagent IV – Hydrolysis of nitriles using strong, hot aquious acid or base RCH2Br Na+ CN(SN2) RCH2C N H3O+ O RCH2COH + NH3 * Excellent two-step process for the preparation of carboxylic acids from 1° halides O CH3 O Br 1. NaCN 2. –OH/H2O 3. H3O C O OH CH3 Fenoprofen (an antiarthritic agent) * Product has one more carbon than the starting alkyl halide V – Carboxylation (or carbonation) of Grignard reagents 20.6 Preparation of Carboxylic Acids Br MgBr H3 C CH3 CO2H H3 C CH3 Mg Ether H3 C CH3 1. CO2, ether 2. H3O+ CH3 CH3 CH3 2,4,6-Trimethylbenzoic acid (87%) 1-Bromo-2,4,6-trimethyl-benzene •Reaction is limited to alkyl halides that can form Grignard reagents (i.e. reactants with specific functional groups) Reaction of Amide with SOCl2 H O R:- + +MgBr +O C O + H C R O O - + MgBr O + H C R OH 20.7 RNX of Carboxylic Acids: An Overview General Reactions of Carboxylic Acids O H H C H - O Deprotonatoin H C OH Reduction O H O R C OH O Carboxylic acid C OX Alpha substitution H C Y Nucleophilic acyl substitution Carboxylic acids can be reduced using two approaches 20.8 Reduction of Carboxylic Acids I – Using LiAlH4 to give 1° alcohols O CH3(CH2)7CH=CH(CH2)7COH Oleic acid 1. LiAlH4, THF 2. H3O+ CH3(CH2)7CH=CH(CH2)7CH2OH cis-9-Octadecen-1-ol (87%) * Reaction usually requires harsh conditions (i.e. heating) II – Using borane (BH3) to give 1° alcohols OH OH C O NO 2 p-Nitrophenylacetic acid 1. BH3, THF 2. H3O+ H NO 2 2-(p-Nitrophenyl)ethanol (94%) * Reaction is usually performed under mild conditions and can be used to selectively reduce carboxylic acid functionality H Preparation of Nitriles The dehydration reaction occurs first with the nucleophilic amide oxygen atom reacting with SOCl2, then a deprotonation of the molecule in a subsequent E2-like elimination reaction. Reaction of Amide with SOCl2 O 20.9 Chemistry of Nitriles S Cl O S S Cl O O R O NH2 R O Cl N H + Cl Base H R R N H Base C N + SO2 Reactions of Nitriles Nitrile groups are strongly polarized, thus resulting in a electrophilic carbon atom. Therefore they are attacked by nucleophiles and yield an sp2-hybridized imine anions. 20.9 Chemistry of Nitriles This reaction is analogous to the formation of an sp3-hybridized alkoxide ion by nucleophilic addition to a carbonyl group. Carbonyl Compound Nu-- δ- O O Products δ+ R R R R Nu Nu-- Nitrile δ- N N - δ+ Products R R Nu Imine anion General Reactions of Nitriles O O H2O NH2 R R H2O Carboxylic Acid Amide 20.9 Chemistry of Nitriles OH N C LiAlH4 H H R R’MgX O Nitrile C R NH2 Amine R R' Ketone Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine O + R C N H3O + Or NaOH, H2O R NH3 OH 20.9 Chemistry of Nitriles Basic hydrolysis of a nitrile R C -- N OH Dianion - O O - OH C R C R OH N - H C N R R R NH2 + NH2 H2O H Amide OH -- - C OH C N + -- H O O OH H H O NH2 - O + C R O - Carboxylate Reduction: Conversion of Nitriles into Amines A nitrile can be reduced with LiAlH4 to give a primary amine C N CH2NH2 1. LiAlH4, ether 2. H2O 20.9 Chemistry of Nitriles CH3 CH3 o-Methylbenzonitrile o-Methylbenzylamine The following example illistrates a reaction that occurs by nucleophilic addition of hydride ion to the polar C≡N bond, yielding an imine anion. The imine anion undergoes another nucleophilic addition to yield a dianion. N R C N LiAlH4 ether LiAlH4 ether C R Nitrile - H Imine anion H H C R H2O 2- N Dianion H H C R NH2 Amine Reaction of Nitriles with Organometallic Reagents A nitrile can add a Grignard reagent to give an intermediate imine anion that is further hydrolyzed by water to yield a ketone: N :R’- +MgX R C O H2O N C R 20.9 Chemistry of Nitriles - Nitrile + C R' Imine anion R NH3 R' Ketone This type of reaction is similar to the reduction of a nitrile to an amine, however only one nucleophilic addition occurs and the nucleophile is a carbanion (R:-) rather than a hydride ion: O C N C CH2CH3 1. CH3CH2MgBr, ether 2. H3O+ Benzonitrile Propiophenone (89%) 20.10 Spectroscopy of Carboxylic Acids Infrared Spectroscopy – Two characteristic IR absorptions I. O-H gives broad band in the range of 2500 – 3300 cm-1 II. C=O gives band in the range 1710 – 1760 cm-1 O O H3 C H O H3 C C O H Monomer CH3 O H Hydrogen-bonded dimer * Position depends on whether the acid exists as a monomer or hydrogen-bonded dimer IR Spectrum of Butanoic Acid O 20.10 Spectroscopy of Carboxylic Acids NMR Spectroscopy • Acidic –COOH proton absorbs as a singlet near 12 δ • Carboxyl carbon atoms absorb in the range 165 - 185 δ • Aromatic / saturated near 165 ∂, aliphatic near 185 δ NMR spectrum of phenylacetic acid