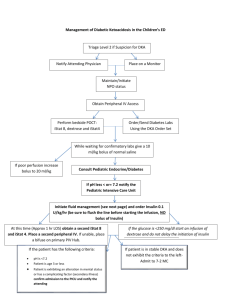
DKA Research Paper - Gabrielle Rutenberg: Dietetic Portfolio
advertisement

Treating DKA in Type 2 Diabetes Case Study 2015 By: Gabrielle Rutenberg DKA Case Study; Gabrielle Rutenberg 1 Abstract Diabetes has become a major epidemic in our country. When poorly controlled many complications can arise such as Diabetic Ketoacidosis (DKA). Typically, this is seen in type 1 diabetics, however, in the last two decades this traditional association has been challenged with increasing reports of DKA in type 2 diabetes. (43) It occurs when the body cannot use glucose as a fuel source because there is no insulin or not enough insulin within the body. When your cells are unable get the glucose they need for energy, your body begins to burn fat. This creates ketones or acids that build up in the blood and urine. In high levels, ketones are poisonous, which make the individual extremely sick. Other reasons why DKA could happen involve steroid medications, infections, or a stressful medical condition in diabetic patients. Three major organs are affected by this disease process including the pancreas, liver, and kidney’s causing symptoms of polyuria, polydispsia, nausea/vomiting, weakness, confusion, abdominals, or shortness of breath. There are different methods for diagnosing DKA and may be different for each individual depending on the severity of their condition. Typically a blood test, urinalysis, chest x-ray, and an electrocardiogram will be done. Treatment of DKA follows a specific protocol, which includes fluid replacements, insulin therapy, and electrolyte replacement. This will stabilize the patients and prevent symptoms from worsening or even death from occurring. While the patient is recovering in the hospital from DKA, this poses a good time to reinforce nutritional guidelines for diabetes and any complications that can arise from the disease. Goals for nutrition therapy that apply to adults with DKA and type 2 diabetes is to educate the patient on carbohydrate consistency, support healthful eating patterns emphasizing a variety of nutrient dense foods in appropriate portion sizes, effects of insulin on the body, and to improve overall health and specifically to attain individualized glycemic, blood pressure, and lipid goals. Being DKA Case Study; Gabrielle Rutenberg 2 able to control your diabetes effectively will prevent complications association with the disease like DKA from occurring. Introduction Diabetes has become a major epidemic in our country within the last decade. The American Diabetes Journal published an article titled, “Projection of Diabetes Burden Through 2050”, which had frightening results. According to this article, the number of Americans with diagnosed diabetes is projected to increase 165%, from 11 million in 2000 to 29 million in 2050! Since 1980 the cases of diagnosis in diabetes has increased 2.9 million. Several factors were taken into account as to why the numbers have increased, which include the aging of our population, sex and race, and obesity/lifestyle. (10) However, The Centers for Disease Control and Prevention came out with current statistics in June of 2014 stating, more than 29 million people in the United States already have type 2 diabetes, and more than one in three U.S. adults have pre-diabetes. (17) These statistics show how quickly diagnoses of type 2 diabetes are increasing. As a future dietitian, these numbers are important when looking at the need for treatment within an outpatient and clinical setting. Our goals are to try to help prevent diseases like diabetes, by advocating healthy diets and increasing the amount of exercise we get each day. However, it is also our duty to be able to treat individuals who have already been diagnosed with this disease and know all the complications that can arise with it. Diabetic ketoacidosis has always been looked at as a major life-threatening emergency in people with poorly managed type 1 diabetes. It occurs when the body cannot use glucose as a fuel source because there is no insulin or not enough insulin within the body. When your cells are unable to get the glucose they need for energy, your body begins to burn fat. This creates ketones, acids that build up in the blood and urine. In high levels, ketones are poisonous, which DKA Case Study; Gabrielle Rutenberg 3 make the individual extremely sick. Due to the increase in type 2 diabetes, we are now seeing an increase between the two. Unfortunately, in the US, incidents of hospitalizations due to DKA have increased. According to Bonnie Sanders Polin, PHD, “Currently, 4% to 9% of all hospital discharge summaries among patients with diabetes include DKA.” (11) The American Diabetes Association circulated an article stating, 138 consecutive admissions for DKA at a large academic center observed that 21.7% had type 2 diabetes. Nearly 70% of the admissions involved discontinuation of medications, and almost half had an identifiable infection when an intensive search was undertaken. (12) A study conducted in Bronx, New York published in the February 2007 issue of Metabolism, found that 32% of the subjects studied had type 2 diabetes with DKA and that it was found primarily in AfricanAmericans and Hispanics. It concluded that African-Americans with type 2 diabetes may be particularly susceptible to developing DKA. (13) However, regardless of race two of the main factors that contribute to this disease state is poor management of diet and medications. With both type 2 diabetes and DKA being on the rise, I chose this topic to gain more insight. I believe a dietitian can really make in difference in the prevention of this lifethreatening complication. I have begun my clinical rotations at Clara Maass Medical Center in November. Within a month I have already seen numerous diagnosis’s of DKA at my hospital, both in type 1 and type 2. I also specifically chose the type 2 diabetes case since it is less frequently seen, which made the topic even more interesting. DKA Case Study; Gabrielle Rutenberg 4 Discussion of the Disease Anatomy and Physiology of Organs Pancreas Diabetic Ketoacidosis effects different organs within the body. The pancreas, the liver and the kidneys are all vital organs that are hugely affected when the body is experiencing DKA. However, first we will look at the anatomy and physiology of these three organs. The pancreas plays an essential role in converting the food we eat into fuel for the body's cells. It is located behind the stomach and is surrounded by other organs including the small intestine, liver, and spleen. It is about six inches long and is shaped like a flat pear. The pancreas has two main functions which include an exocrine function that helps digestion and an endocrine function that regulates blood sugar. (5) The exocrine function involves small glandular cells that produce digestive juices making about 1.5 to 2 liters per day, which are then released into the intestines. These gastric juices are mainly made up of water, salt, sodium bicarbonate and digestive enzymes. Sodium bicarbonate neutralizes the acidic gastric juice of the semi-digested food, ensuring that the digestive enzymes can work at their optimal level. This gastric juice produced by the pancreas also helps to break down protein, sugar, and fats. The Institute for Quality and Efficiency in Health Care explains that between the glandular cells that produce the digestive juice, there are tiny collections of different glandular cells called islets. (1) These islet cells produce insulin, glucagon and other hormones and release them into the bloodstream within our bodies. These hormones make sure that the blood sugar levels are neither too high nor too low. When the blood sugar level rises following a meal that was consumed the pancreas releases insulin. Insulin enters the bloodstream and ensures that the sugar in the food and drinks we consume is transported from our blood to our cells, where it is then transformed into energy for DKA Case Study; Gabrielle Rutenberg 5 the body. This process is known as the endocrine process within the pancreas. The maintenance of keeping blood sugar levels at a proper level is crucial to the functioning of other organs in our bodies including the brain, liver, and kidneys. Liver The liver is located in the upper right-hand portion of the abdominal cavity. It is a dark reddish-brown organ that has multiple functions. The liver regulates most chemical levels in the blood and excretes a product called bile. Bile helps to break down fats, preparing them for further digestion and absorption. All of the blood leaving the stomach and intestines passes through the liver, which there it will process the blood and break it down, balance it, and create nutrients for the body to use. It also metabolizes drugs in the blood into forms that are easier for the body to use. The liver also acts as the body’s glucose reservoir, and helps to keep the circulating blood sugar levels and other body fuels steady and constant. The liver both stores and manufactures glucose depending upon the body’s need. The need to store or release glucose is primarily signaled by the hormones insulin and glucagon. (6) Insulin causes the liver and the muscles to store sugar, and inhibits new production of sugar in the liver. However, when blood sugar levels decrease, the pancreas releases glucagon into the bloodstream. Glucagon is a peptide hormone, produced by alpha cells of the pancreas, which raises the concentration of glucose in the bloodstream. Its effect is opposite of insulin, which lowers the glucose concentration. Glucagon also ensures that the cells of the liver produce new sugar from other substances in the body. When the blood sugar level has risen, the release of glucagon is stopped once again. (1) Kidneys DKA Case Study; Gabrielle Rutenberg 6 In addition to the pancreas and liver, the kidneys are also effected by DKA. The kidney’s responsibility is to act as the waste filtering and disposal system of the body. As much as 1/3 of all blood leaving the heart passes into the kidneys to be filtered before flowing to the rest of the body’s tissues. Our bodies have two kidneys, which filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine, composed of wastes and extra fluid every day. The urine flows from the kidneys to the bladder through two thin tubes of muscle called ureters, one on each side of the bladder. The bladder stores urine. The muscles of the bladder wall remain relaxed while the bladder fills with urine. As the bladder fills to capacity, signals sent to the brain tell a person to find a toilet soon. When the bladder empties, urine flows out of the body through a tube called the urethra, located at the bottom of the bladder. (4) The liver and the kidneys also work together to further filter the body. The liver metabolizes dietary proteins to produce energy and produces toxic ammonia as a waste product. The liver is able to convert most of this ammonia into uric acid and urea, which are less toxic to the body. Meanwhile, the muscles of our body use creatine as an energy source and produce the waste product creatinine in the process. Ammonia, uric acid, urea, and creatinine all accumulate in the body over time and need to be removed from circulation to maintain homeostasis. This is where the function of the kidney’s come in. The glomerulus in the kidneys filter all four of these waste products out of the bloodstream, allowing us to excrete them out of our bodies in urine. The kidney’s also help keep levels of electrolytes stable, such as sodium, potassium, and phosphate, make hormones to help regulate blood pressure, make red blood cells, and help keep our bones strong. Disease process Diabetic ketoacidosis arises because of a lack of insulin in the body, due to the pancreas either not producing it or our bodies resisting the effects of insulin. The lack of insulin and DKA Case Study; Gabrielle Rutenberg 7 corresponding elevation of glucagon leads to an increased release of glucose by the liver from glycogen via glycogenolysis and also through gluconeogenesis. The increased rate of glucose production in the liver, coupled with the glucagon-mediated inhibition of glucose storage into glycogen results in the increased glucose release from the liver and consequent hyperglycemia. (2) The absence of insulin also leads to the release of free fatty acids from adipose tissue, which are then converted in the liver into ketone bodies called acetoacetate and β-hydroxybutyrate. βHydroxybutyrate can serve as an energy source in the nonexistence of insulin, and is a protective mechanism in case of starvation. On the other hand, ketone bodies have a low pH, causing the blood acidity to increase producing metabolic acidosis. (3) In addition, as the blood sugar levels rise, the kidneys cannot retain the extra sugar, which is dumped into the urine, thereby increasing urination and causing dehydration. About 10% of total body fluids are lost as the patient slips into diabetic ketoacidosis. (7) Although DKA is normally found in type 1 diabetes, individuals with type 2 diabetes can also experience this disease state. Excess ketone production happens for a number of reasons. Maybe the individual did not inject enough insulin or missed their dose of insulin completely. Not enough food can also be an issue, as well as having a reaction to the insulin that was injected. Other reasons could involve steroid medications, infections, or a stressful medical condition. Symptoms There are many symptoms that arise when an individual is experiencing diabetic ketoacidosis. Nausea and vomiting are a common sign. As ketones accumulate in the blood, more ketones will be passed in the urine, taking sodium and potassium salts out with them. Over time, the levels of sodium and potassium salts in the body become depleted, which can cause DKA Case Study; Gabrielle Rutenberg 8 nausea and vomiting. Shortness of breath is another DKA symptom. As the body's natural buffering system is overwhelmed by the acidic ketones, this imbalance causes hyperventilation as the body attempts to regulate blood acid levels by getting rid of carbon dioxide in expired air. Since the body is trying to get rid of the excess acid by exhaling it a persons breathe can have a fruity-like smell. Your body is also not receiving its normal source of energy resulting in extreme fatigue and weakness. When glucose becomes hyper-concentrated in your bloodstream, your kidney lose the ability to pull the glucose out from water. The osmotic pressure builds up getting so high that water can no longer be absorbed back into your bloodstream causing dehydration and polydipsia. Again, the high ketone levels are associated with the high sugar levels in the blood and urine. More water is drawn into the urine due to the high ketone levels associated with the high sugar levels in blood and urine resulting in polyuria. This combined with vomiting causes the body to quickly lose too much water and electrolytes also resulting to dehydration. Methods of Diagnosis There are different methods for diagnosing DKA and may be different for each individual depending on the severity of their condition. Typically a blood test, urinalysis, chest x-ray, and an electrocardiogram will be done. An MRI can also be helpful in detecting early cerebral edema, however it should be ordered only if altered consciousness is present. First, a blood test may be done to check glucose levels, pH, bicarbonate, ketones, anion gap, electrolyte panel, and CBC. Glucose levels may be looked at to see if levels have reached 250 mg/dL or higher, which is a marker for hyperglycemia. The blood tests also checks the arterial blood gas levels, which checks the pH levels. If levels are <7.3 then the blood is too acidic. Serum bicarbonate levels <15 mEq/L is also a parameter indicative of acidosis. Ketones can be checked with both the DKA Case Study; Gabrielle Rutenberg 9 urine and blood. Urinary ketones >3 or if serum ketones are positive this is an indication of ketosis. The anion gap is the difference between primary measured cations, sodium, and potassium, as well as the primary measured anions chloride and bicarbonate in serum. In patients with diabetic ketoacidosis, the anion gap is elevated greater than 10 mEq/L in mild cases and greater than 12 mEq/L in moderate and severe cases. A serum electrolyte panel will also be taken to also check acid-base imbalance. Serum potassium levels initially are high or within the reference range in patients with DKA. This is due to the extracellular shift of potassium in exchange of hydrogen, which is accumulated in acidosis, in spite of severely depleted total body potassium. This needs to be checked frequently, as values drop very rapidly with treatment. The serum sodium level usually is low in affected patients. The osmotic effect of hyperglycemia moves extravascular water to the intravascular space. For each 100 mg/dL of glucose over 100 mg/dL, the serum sodium level is lowered by approximately 1.6 mEq/L. When glucose levels fall, the serum sodium level rises by a corresponding amount. Additionally, serum chloride levels and phosphorus levels always are low in these patients. (7) Even in the absence of infection, the CBC count shows an increased white blood cell count in patients with diabetic ketoacidosis. BUN is also increased as a result of dehydration. DKA may be precipitated by a cardiac event, and the physiological disturbances of DKA may cause cardiac complications. An ECG should be performed every 6 hours during the first day, unless the patient is monitored. An ECG may reveal signs of acute myocardial infarction that could be painless in patients with diabetes, particularly in those with autonomic neuropathy. An ECG is also a rapid way to assess significant hypokalemia or hyperkalemia. T-wave changes may produce the first warning sign of disturbed serum potassium levels. Low T wave and apparent U wave always signify DKA Case Study; Gabrielle Rutenberg 10 hypokalemia, while peaked T wave is observed in hyperkalemia. Sometimes a doctor will want to perform a chest x-ray to rule our pneumonia or any other pulmonary infections. (7) Treatment of DKA Fluid Replacement Treatment of DKA should be handled very carefully and monitored frequently. According to an article written in the American Diabetes Association journal, successful treatment of DKA requires correction of dehydration, hyperglycemia, and electrolyte imbalances, identification of comorbid precipitating events, and frequent patient monitoring. (3) Initial fluid therapy is directed toward expansion of the intravascular, interstitial, and intracellular volume, all of which are reduced in hyperglycemic crises. In the absence of cardiac compromise, isotonic saline (0.9% NaCl) is infused at a rate of 15–20 ml/kg body weight during the first hour. Typically, the choice for fluid replacement depends on hemodynamics, the state of hydration, serum electrolyte levels, and urinary output. In general, 0.45% NaCl infused at 250– 500 ml/h is appropriate if the corrected serum sodium is normal or elevated and 0.9% NaCl at a similar rate is appropriate if corrected serum sodium is low. To see if progress is being made with fluid replacement a nurse or doctor may check blood pressure, measurements of fluid input/output, laboratory values, and do a clinical examination. Ideally, fluid replacement should correct deficits within the first 24 hours. In patients with renal or cardiac compromise, monitoring of serum osmolality and frequent assessment of cardiac, renal, and mental status must be performed during fluid resuscitation to avoid too much fluid. The American Diabetes Journal states, an aggressive rehydration with subsequent correction of the hyperosmolar state has been shown to result in a more robust response to low-dose insulin therapy. (3) Patients who start treatment for DKA correct hyperglycemia faster than ketoacidosis. The mean duration of DKA Case Study; Gabrielle Rutenberg 11 treatment until blood glucose is <250 mg/dl and ketoacidosis is corrected is about 6-12 hours. Once the plasma glucose is down to 200 mg/dl, 5% dextrose should be added to replacement fluids to allow continued insulin administration until ketonemia is controlled while at the same time avoiding hypoglycemia.(3) Insulin Therapy The next step to stabilize a patient with DKA is insulin therapy. All oral diabetic medications and other insulin’s should be discontinued. Regular insulin, which is short-acting, should be given by intravenous infusion or by frequent subcutaneous/intramuscular injections. Patients can either receive insulin IV bolus of 10 units (2) or an hourly insulin infusion of 0.14 units/kg body weight. (3) Low-dose insulin infusion protocols decrease plasma glucose concentration at a rate of 50–75 mg/dl. If plasma glucose does not decrease by 50–75 mg from the initial value in the first hour, the insulin infusion should be increased every hour until a steady glucose decline is achieved. (3) Once the glucose level decreases to 200 mg per dL, the insulin infusion rate should be decreased to 0.05 to 0.1 units/kg/hour, and D5 should be added to the intravenous fluids to maintain a glucose level between 150 and 200 mg per dL. The blood glucose level should not be allowed to fall lower than 200 mg/dL during the first 4-5 hours of treatment. To prevent recurrence of hyperglycemia or ketoacidosis during the transition period to subcutaneous insulin, it is important to allow an overlap of 1–2 h between discontinuation of intravenous insulin and the administration of subcutaneous insulin. If the patient is to remain fasting/nothing by mouth, it is preferable to continue the intravenous insulin infusion and fluid replacement. Patients with known diabetes may be given insulin at the dosage they were receiving before the onset of DKA so long as it was controlling glucose properly. (3) Electrolyte Replacement DKA Case Study; Gabrielle Rutenberg 12 Potassium levels should be monitored every two to four hours in the early stages of DKA. Insulin therapy, correction of acidosis, and volume expansion decrease serum potassium concentration. To prevent hypokalemia, potassium replacement is initiated after serum levels fall below the upper level of normal for the particular laboratory. The treatment goal is to maintain serum potassium levels within the normal range, which is 4–5 mEq/l. Generally, 20–30 mEq potassium in each liter of infusion fluid is sufficient to maintain a serum potassium concentration within the normal range. If a patient is experiencing hypokalemia, potassium replacement should begin with fluid therapy, and insulin treatment should be delayed until potassium concentration is restored to >3.3 mEq/l to avoid life-threatening arrhythmias and respiratory muscle weakness. (3) However, if urine output is <30 mL/hr then potassium replacement should be put on hold. Another part of the initial treatment of DKA includes the need for bicarbonate. This may be different for each patient depending on their pH levels assessed by the arterial blood gas measurement. If the pH <6.9 give 100mEq sodium bicarbonate in 1L D5W and infuse at 200ml/hr. If the pH is 6.9 – 7.0 give 50mEq sodium bicarbonate in 1L D5W and infuse at 200ml/hr. This should be continued until pH is >7.0. However if the pH is already over 7.0 then this step can be avoided. (2) DKA is resolved when the plasma glucose is below 200, serum bicarbonate is <18, blood pH is >7.3, and the anion gap is <10. Complications/Comorbidities The American Diabetes Journal, displayed an article titled, “Hyperglycemic Crises in Adult Patients with Diabetes”. It discusses the complications that may arise when treating DKA. Hypoglycemia and hypokalemia are two common complications with the over treatment of DKA with insulin and bicarbonate, but these complications have occurred less often when low-dose DKA Case Study; Gabrielle Rutenberg 13 insulin therapy is used. Cerebral edema is another complication, which occurs in about 0.3–1.0% of DKA episodes in children. However, it is extremely rare in adult patients during treatment of DKA. Cerebral edema is associated with a mortality rate of 20–40% and accounts for 57–87% of all DKA deaths in children. (3) Ketoacidosis may also impair nerve function and may cause gastro paresis. (25) The American College of Gastroenterology suggests, markedly uncontrolled glucose levels (>200 mg/dL), may aggravate symptoms of gastro paresis and delay gastric emptying. (45) Current Research A recent study was conducted and published in the American Diabetes Journal called, “Treatment of diabetic ketoacidosis with subcutaneous insulin lispro: a review of the current evidence from clinical studies.” Its aim was to see if low-dose IV of regular insulin is needed in initial treatment of DKA. There were four small randomized studies done that included 156 patients overall. Three studies were done in adults and one in pediatric patients with diabetes. One group received subcutaneous insulin lispro injections every 1-2 hours, while the other group received the standard IV of regular insulin. Patients with severe complications were not included in the study. The conclusion of the study indicated that patients with mild to moderate case of diabetic ketoacidosis can receive subcutaneous insulin lispro an alternative to the IV of regular insulin. This will also decrease the need for ICU admissions. (9) The article previously mentioned and published by the American Diabetes Journal, titled, “Hyperglycemic Crisis in Adult Patients with Diabetes”, also states that treatment with subcutaneous rapid-acting insulin analogs like lispro or aspart, has been shown to be an effective alternative to the use of IV regular insulin in the treatment of DKA. However, it also states that if cases are severe the patient should stick to IV insulin only. (9) DKA Case Study; Gabrielle Rutenberg 14 Medical Nutrition Therapy Medical nutrition therapy for patients with DKA is initiated once the patient is stabilized within the intensive care unit. Typically patients will feel much better within 12-14 hours of treatment. Depending on whether they have any other underlying conditions, a patient is able to start on a diet within 12-24 hours. However, if they are still feel unwell, then a combination of parenteral 5 or 10% glucose and hypo caloric liquid feeding is given. While the patient is recovering in the hospital from DKA, this poses a good time to reinforce nutritional guidelines for diabetes and any complications that can arise from the disease. For a patient with type 2 diabetes, weight management, blood glucose control, education about the effects of insulin, and other several chronic conditions such as heart disease, and hypertension play an imperative role in the management of the disease. An interdisciplinary team is needed to integrate MNT into the overall management plan and prevention of complication such as DKA. Diabetes nutrition selfmanagement education, although potentially initiated in the hospital, is usually best provided in an outpatient or home setting where the individual with diabetes is better able to focus on learning needs. (27) Goals for nutrition therapy that apply to adults with DKA and type 2 diabetes is educate the patient on carbohydrate consistency, support healthful eating patterns emphasizing a variety of nutrient dense foods in appropriate portion sizes, effects of insulin on the body, and to improve overall health and specifically to attain individualized glycemic, blood pressure, and lipid goals. General recommended goals from the ADA for these markers are as follows: Marker Goals HbA1c <7% DKA Case Study; Gabrielle Rutenberg 15 Blood Pressure <140/80 mmHg LDL <100 mg/dL HDL >40 mg/dL (men); >50 mg/dL (women) Triglycerides <150 mg/dL Table 1.1 (32) Nutrition Assessment A thorough nutrition assessment of the patient is needed in order to determine the best nutrition intervention and plan of care for the patient. This would include anthropometric measurements such as height and weight of the individual. It is important to gather weight loss history, especially if there has been recent weight loss or weight gain that may be of concern. Looking at usual body weight vs. current body weight can help determine this. More than 5 percent of weight loss or weight gain in six months to a year can be a red flag of malnutrition, over nutrition, or an underlying medical condition. Biochemical data is also looked at to examine any abnormal laboratory values. Pertinent labs to review include: Labs Normal DKA Blood glucose 70-110 mg/dL >250 mg/dL Sodium 135-145 mEq/L <135 mEq/L Potassium 3.5-5.1 mEq/L >3.5 mEq/L Phosphorous 2.4-5.1 mEq/L <2.3 mEq/L Chloride 98-110 mEq/L <97 mEq/L Serum bicarbonate 22-26 mEq/L <18 mEq/L BUN 7-18 mg/dL >18 mg/dL DKA Case Study; Gabrielle Rutenberg 16 Creatinine 0.8-1.30 mg/dL >1.30 mg/dL Blood pH 7.34-7.44 <7.2 Anion gap <10 mEq/L 10-12 mEq/L Blood/Urine ketones negative positive White Blood Cell 4,300 - 10,800 >11,000 Table 1.2 Diagnostic tests should be looked at as well. These include blood tests to determine the pertinent laboratory values as mentioned above, chest x-ray to rule out respiratory conditions, and an ECG to look at any cardiac complications. An ECG is also a rapid way to assess significant hypokalemia or hyperkalemia as mentioned above in the disease section. An MRI may also be ordered if altered consciousness is present to detect cerebral edema. Looking at past medical history may help determine if the patient has experienced DKA once before and any other disease states the patient may have. It is also important to look at this section to determine what type of diet the patient should be consuming, as well as calculating their nutritional needs. In regards to their diet and social history, this will help determine what type of education the patient will need. Looking at what foods are typically consumed and what their living situation is can paint a picture on how to gear their initial consultation. A study was done by Bruna Galobardes, Alfredo Morabia, and Martine S Bernstein called, “Diet and socioeconomic position: does the use of different indicators matter?” It presented results that showed that lower education and lower occupation independently contribute to determining differences in dietary habits and that the effect of the two indicators is cumulative. For instance, men from lower socioeconomic position consumed less fish and vegetables, but more pasta, potatoes, fried foods, table sugar, and beer. Women of lower socioeconomic position also DKA Case Study; Gabrielle Rutenberg 17 consumed less fish and vegetables, but more meat, fried foods, table sugar, pasta, and potatoes. Lower intake of iron, calcium, vitamin A and vitamin D was present among lower socioeconomic groups as well. (24) These results are important because it shows people of a lower economic status may be at a higher risk of DKA or other disease states, due to their higher consumption of processed foods and lower consumption of fresh fruits and vegetables. These individuals may also not be able to afford their medications, which is a huge cause of DKA. Looking at their physical appearance is another important factor in completing a full assessment. Observing to see if the patient looks obese or frail, their hygiene, dental status, amputations, or wounds will help determine caloric needs and diet consistency modifications. Calorie Needs To achieve near normal blood glucose levels, people with type 2 diabetes who are taking insulin or oral medication must coordinate calorie intake with their medication or insulin administration, exercise, and other variables to control blood glucose levels. (26) This is important in preventing complications, such as DKA. To determine calorie needs, the patients BMI, age, and disease state must be looked at. For most patients the caloric range will be 25–35 kcal/kg per day. Care must be taken not to overfeed patients because this can exacerbate hyperglycemia. (27) James Norman MD states, patients with type 2 diabetes generally are put on a 1,500 to 1,800 calorie diet per day to promote weight loss and then the maintenance of ideal body weight. However, this may vary depending on the person's age, sex, activity level, current weight, and body styles. More obese individuals may need more calories initially until their weight is less. This is because it takes more calories to maintain a larger body, and a 1,600 calorie diet for them may promote weight loss that is too fast to be healthy. (45) Macronutrients DKA Case Study; Gabrielle Rutenberg 18 Carbohydrates The University of Maryland Medical Center, explains that carbohydrates should provide 45 - 65% of total daily calories for someone living with type 2 diabetes. The type and amount of carbohydrates are both important for someone living with type 2 diabetes and the best choices include vegetables, fruits, beans, and whole grains. (26) In contrast, The American Diabetes Association suggests, there is no single meal planning system that is ideal for hospitalized patients. However, it is suggested that hospitals consider implementing a consistent-carbohydrate diabetes meal-planning system. This systems uses meal plans without a specific calorie level, but consistency in the carbohydrate content of meals. The carbohydrate contents of breakfast, lunch, dinner, and snacks may vary, but the day-to-day carbohydrate content of specific meals and snacks is kept constant. In diabetes management, it is important to match doses of insulin and insulin secretagogues to the carbohydrate content of meals. A variety of methods can be used to estimate the nutrient content of meals, including carbohydrate counting and experience-based estimation. (27) The nutrition care manual explains that carbohydrate intake should be kept consistent on a day-to-day basis to ensure glycemic control and insulin doses should match carbohydrate intake. This is called insulin-to-carbohydrate ratio. (33) Protein For individuals with type 2 diabetes and normal renal function, there is insufficient evidence to suggest that usual protein intake of 15–20% of energy should be modified. (33) Ingesting protein can increase insulin response without increasing plasma glucose DKA Case Study; Gabrielle Rutenberg 19 concentrations, therefore it is a safe meal choice for patients experiencing hyperglycemia. However, long-term effects of protein intake >20% of calories on diabetes management and its complications are unknown. Small, short-term studies in diabetes suggest that diets with protein content >20% of total energy reduce glucose and insulin concentrations, reduce appetite, and increase satiety. However, the effects of high-protein diets on long-term regulation of energy intake, satiety, weight, and the ability of individuals to follow such diets long term have not been adequately studied. (27) When combined with carbohydrates, protein can increase the body’s ability to respond to insulin, there by maintaining blood glucose control. (29) Fat Type 2 diabetes can make an individual more susceptible to obtain heart disease, so limiting fat, especially saturated fat and artificial trans fats is key. (30) Patients with diabetes often have unhealthy cholesterol levels including high LDL cholesterol, low HDL cholesterol, and high triglycerides. This triad of poor lipid counts often occurs in patients with premature coronary heart disease. It is also a characteristic of a lipid disorder associated with insulin resistance called atherogenic dyslipidemia, or diabetic dyslipidemia in those patients with diabetes. (31) Fats should provide 25 - 35% of daily calories in patients with type 2 diabetes. However, sources should include monounsaturated (such as olive, peanut, canola oils, avocados and nuts) and omega-3 polyunsaturated (such as fish, flaxseed oil, and walnuts). Saturated fat should be less than 7% of daily calories and trans-fats (such as hydrogenated fat found in snack foods, fried foods, and commercially baked goods) to less than 1% of total calories. Like the theory that certain carbohydrates can protect against heart disease and control blood sugar, Dr Hu also explains that the type of fat may be more important than the amount of fat a person DKA Case Study; Gabrielle Rutenberg 20 consumes. (19) This is due to that fact that these types of fats help lower triglycerides, lower cholesterol, lower LDL’s and increase HDL’s. (18) Fiber Dietary fiber has been proved to be beneficial in controlling blood sugar levels. When fiber is digested, your body handles it differently than the way in which refined carbohydrates, such as white flour, are digested. A portion of the fiber simply passes through your digestive system intact. This difference means that eating foods rich in fiber is less likely to cause a spike in high blood sugar. Many foods high in fiber are also rich in antioxidants. Fiber also helps with hunger control, keeping us full longer which creates less of a chance that a person will consume foods that will spike blood sugar. A study in the New England Journal of Medicine showed that people with diabetes who ate 50 grams of fiber a day, particularly soluble fiber, were able to control their blood glucose better than those who ate far less. (23, 33) The recommended daily amount of fiber is 25 grams for women and 38 grams for men. Micronutrients Sodium High blood pressure has long been recognized as a major risk factor for cardiovascular disease. Studies report a positive association between hypertension and insulin resistance. When patients have both hypertension and diabetes, which is a common combination, their risk for cardiovascular disease doubles. (31) A recent analysis of data from more than 4,000 adults in the Framingham Heart Study showed that even though people with diabetes have done a better job of managing heart disease risk factors over the past few decades, they need to do more to protect heart health, including lowering high blood pressure. People with diabetes are still two to three DKA Case Study; Gabrielle Rutenberg 21 times more likely to develop heart disease than those without heart disease, according to the study. The American Diabetes Association recommends that people with diabetes aim to have 2300 mg or less per day. If any individual already has high blood pressure, the recommendations will be lower. (20) However, Sacha Uelmen RD proposes that the recommended salt intake for someone with diabetes or other heart risk factors should be less than 1,500 mg a day. (40) Magnesium Magnesium contributes to many functions within our bodies. Its makes up our teeth and bone, activates enzymes, regulates protein synthesis, muscle and nerve function, blood glucose control, and blood pressure. Magnesium can also prevent of atherosclerosis, and prevent of retinopathy, a side effect associated with poorly managed diabetes. (34) Individuals with diabetes are more likely than those without to be low in magnesium. Elevated blood glucose levels increase the loss of magnesium in the urine, which in turn lowers blood levels of magnesium. (41) A study was done with nine randomized double-blind controlled trials with a total of 370 patients with type 2 diabetes for a duration 4-16 weeks. The treatment group was supplemented with 360 mg of magnesium per day. The outcome of the study showed a positive reduction in plasma fasting glucose levels, while raising HDL cholesterol in patients with type 2 diabetes. (42) With DKA, urinary loss of magnesium particularly during intensive insulin and fluid therapy can be seen. This loss is reported in more than 55% of patients after initiation of therapy, leading to hypomagnesaemia. On the other hand some studies show raised magnesium level in diabetic patients with poor renal function. Using magnesium supplementation can also help prevent arrhythmia's, a complication associated with DKA. The Recommended Daily Allowance (RDA) for magnesium is 6 mg/kg/d. This means 400 mg/d to 420 mg/d for adult men and 320 mg/d for adult women. DKA Case Study; Gabrielle Rutenberg 22 Phosphate Despite whole-body phosphate deficits in DKA that average ∼1.0 mmol/kg body weight, serum phosphate is often normal or increased at presentation, however phosphate concentrations will decreases with insulin therapy. Prospective randomized studies have failed to show any beneficial effect of phosphate replacement on the clinical outcome in DKA and overdoing phosphate therapy can cause severe hypocalcemia with no evidence of tetany. On the other hand, to avoid cardiac and skeletal muscle weakness and respiratory depression due to hypophosphatemia, careful phosphate replacement may sometimes be indicated in patients with cardiac dysfunction, anemia, or respiratory depression and in those with serum phosphate concentration <1.0 mg/dl. When needed, 20–30 mEq/l potassium phosphate can be added to replacement fluids. (37) Enteral/Parenteral Nutrition Managing PN in patients at risk of DKA involves daily monitoring of plasma glucose, electrolytes, and fluid balance. According to Sandra I. Austhof, MS, RD, LD, CNSD, and Monica A. Habib, MS, RD, LD, CNSC, PN nutrition should be initiated when plasma glucose levels are under control (<180 mg/dl). When beginning PN, dietitians should restrict the number of carbohydrates given to 100 to 150 g. Research has suggested limiting dextrose administration to 1.5 to 2 mg/kg of body weight per minute. RDs should ensure that regular insulin and hypoglycemia orders are in place and signed by the physician as a safety net before initiating PN to manage possible severe blood sugar highs and lows, which are detrimental to patients. A nurse should monitor capillary blood glucose levels every six hours until they’re consistently less than 150 mg/dL. Since regular insulin is compatible with PN formula, a nurse will add it as needed. Nurses can determine the initial dose by using 0.1 units of regular insulin per gram of dextrose DKA Case Study; Gabrielle Rutenberg 23 given. They can base the amount of insulin given in subsequent bags on two-thirds of the total amount of insulin administered the previous day. (ie. if a nurse gave a patient 16 units of slidingscale insulin on day 1, the nurse would add 10 units to PN on day 2. If hyperglycemia continues after exceeding 0.3 units of regular insulin per gram of dextrose, a nurse would consider adding a separate IV insulin infusion. However, an IV bolus of insulin shouldn’t be given, as severe hypokalemia may quickly develop because it’s short acting. Long-acting insulin, such as glargine, administered subcutaneously as a basal dose, has been shown to improve glycemic control in diabetes patients receiving PN. Dietitians can advance dextrose calories only after glucose levels are well controlled. If the PN contains insulin, it’s important to maintain the ratio of PN insulin to dextrose when increasing or decreasing dextrose calories. If hyperglycemia persists despite daily increases in insulin, RDs can lower dextrose calories and replace them with fat calories (using a three-in-one formulation). However, recent studies have demonstrated that the infusion of fat emulsion increases free fatty acids, leading to insulin resistance and impaired glucose tolerance. In addition, RDs should stop PN when serum glucose becomes greater than 400 mg/dL and replace it with IV normal saline infusion started at the same rate. Also, additional chromium should be included in the PN if a deficiency is present since chromium regulates insulin action. (38) When commencing an enteral feed on a diabetic patient, hyperglycemia can occurs within a few hours and if no insulin treatment is started patients can become grossly polyuric and dehydrated, and may progress to HONK (hyper-osmolar nonketosis) or DKA. IV insulin sliding scale infusion should be started on enteral feeding. Blood glucose monitoring should be done every one to two hours if results outside the normal range of 4-10mmols/litre. It is often useful to continue IV sliding scale for a few days while the enteral feeding rate is progressively DKA Case Study; Gabrielle Rutenberg 24 increased until full feeding rate is achieved. Once full feeding rate is achieved and patient stable the insulin treatment can be converted to a subcutaneous regimen to suit the feeding regimen. Dietitians should directly work with the medical team to agree on the most suitable feeding regimen for the patient. A variety of insulin preparations and regimens may be employed to suit the individual patient’s insulin sensitivity and feeding duration. (39) Another aspect to consider when enteral feeding is necessary is the formula to provide to the patient. A systematic review was looked at with a total of 23 studies, comprising 784 patients of both oral supplements (16 studies) and tube feeding (7 studies). The results showed that compared with standard formulas, diabetes-specific formulas significantly reduced postprandial rise in blood glucose, peak blood glucose concentration, and a reduced requirement for insulin. If such nutritional support is given long term, this may have implications for reducing chronic complications of diabetes, such as cardiovascular events. (40) Education/Prevention Carbohydrate Counting Carbohydrate counting assists the individual to keep track of how much carbohydrate you are eating per meal, and with the right balance of physical activity and medicine it can help to keep your blood glucose levels at a target range. Another benefit of counting carbohydrates is that it can bring tighter control over glucose readings. Being as precise as possible with your carbohydrate intake and medication will help you better manage your blood glucose after meals. For instance, if you take mealtime insulin, counting carbohydrates allows you to create a meal or snack with the correct amount of carbohydrates. (28) Teaching a patient with DKA this system will help control blood glucose levels along with their daily insulin doses. The nutrition care DKA Case Study; Gabrielle Rutenberg 25 manual illustrates how many servings of carbohydrates are needed depending on how many calories are needed within a day: Approximate Energy (kcal) Snack Servings per Day 1,200-1,500 Meal Carbohydrate Servings 3 (45g) 1,600-2,000 4 (60g) 2-3 (30-45g) 2,100-2,400 5 (75g) 4-6 (60-90g) 1 (15g) Table 1.3 (33) Glycemic Index Frank B. Hu, MD, PhD, of the Harvard School of Public Health explains, that the type of carbohydrates you eat is more crucial in monitoring blood sugar levels than the amount eaten. Carbohydrates were formerly classified as simple and complex. The new classification is based on the glycemic index, which ranks food based on their ability to increase blood glucose. For example, glucose has a GI of 100, baked potatoes have a GI of 93, carrots 49, pasta 39, and peanuts 14. Dr. Hu suggested that using the GI, or another similar measure called the glycemic load, could help control diabetes. The data from the Nurses' Health Study suggest that a high dietary glycemic load from refined carbohydrates increases the risk of Coronary Heart Disease, whereas an increased intake of whole grains may protect against CHD. (19) Carbohydrate sources should be taken from lower glycemic index foods such as, whole grains, fresh fruits, and low-fat dairy products, rather than high glycemic index foods like refined sugars, white breads/pastas/rice, candy, and soda. On the other hand, the nutrition care manual explains that use of the glycemic index has mixed results effects on HbA1C levels. (33) Insulin and mealtimes DKA Case Study; Gabrielle Rutenberg 26 Patients with diabetes must be educated on the different types of insulin and its effects on carbohydrates. For instance, the bolus dose for food coverage is prescribed as an insulin to carbohydrate ratio. The insulin to carbohydrate ratio represents how many grams of carbohydrate are covered by 1 unit of insulin. Rapid acting insulin will peak in the blood in about 15-20 minutes, and clears the body in 2-3 hours. Generally, one unit of rapid-acting insulin will dispose of 12-15 grams of carbohydrate. (35) Premixed insulins are generally taken two or three times a day before mealtime and are a combination of specific proportions of intermediate-acting and short-acting insulin in one bottle or insulin pen. In general, you should coordinate your insulin injection with the times you plan to have your meals. You want the insulin to begin working in your body at the same time your food is being absorbed. This timing will help avoid low blood sugar levels, as well as high blood sugar levels if not enough insulin is injected. The table on the next page shows the different types of insulins, their durations, and their role in blood sugar control. DKA Case Study; Gabrielle Rutenberg 27 Types of insulin and brand names Onset Peak Duration Role in blood sugar management Humalog or lispro 15-30 min. 30-90 min. 3-5 hours Rapid-acting insulin covers insulin needs for meals eaten at the same time as the injection. Novolog or aspart 10-20 min. 40-50 min. 3-5 hours Apidra or glulisine Short-Acting 20-30 min. 30-90 min. 1-21/2 hours Regular (R) humulin or novolin 30 min.-1 hour 2-5 hours 5-8 hours Velosulin (for use in the insulin pump) IntermediateActing NPH (N) 30 min.-1 hour 2-3 hours 2-3 hours 1-2 hours 4-12 hours 18-24 hours Intermediateacting insulin covers insulin needs for about half the day or overnight. It is used to control the blood sugar overnight, while fasting and between meals. 1-1½ hour No peak time; insulin is delivered at a steady level 20-24 hours Long-acting insulin covers insulin needs for about one full day. It is used to control the blood sugar overnight, while fasting and between meals. Rapid-Acting Short-acting insulin covers insulin needs for meals eaten within 30-60 minutes. Long-Acting Lantus (insulin glargine) DKA Case Study; Gabrielle Rutenberg 28 Levemir (insulin detemir) 1-2 hours 6-8 hours Up to 24 hours Table 1.4 (35) Glucose/Ketone Monitoring Monitoring your blood sugar levels will tell you how your body is responding to certain foods, activities, and medications, and will help you manage your meal and exercise plans. The American Diabetes Association suggests, to check glucose levels before a meal (preprandial plasma glucose) and after the meal (Postprandial plasma glucose). Before consuming any type of food, glucose levels should read 70-130 mg/dl and after the meal should read <180 mg/dl. It is also recommended to check blood sugars more frequently at a time of stress or illness, since during these times you may forget to eat or take your insulin, which can cause an episode of DKA. (36) It is not necessary to monitor ketone levels daily. However, the American Diabetes Association suggest times that it is imperative to do so when: blood glucose is >240 mg/dl, you feel nauseated, are vomiting, or have abdominal pain you are sick (for example, with a cold or flu) You feel tired all the time You are thirsty or have a very dry mouth Your skin is flushed You have a hard time breathing Your breath smells "fruity" You feel confused or "in a fog" DKA Case Study; Gabrielle Rutenberg 29 Small or trace amounts of ketones may mean that ketone buildup is starting and should be tested again in a few hours. Moderate or large amounts are a danger sign and show that the chemical balance of your blood is too acidic and can poison the body. These are signs that your diabetes is out of control and your body might be going into diabetic ketoacidosis. (37) Sick Days Going over “sick days” with a patient with type 2 diabetes is important in the prevention of DKA. When a person becomes sick their body goes under a great deal of stress. To deal with this stress, the body releases hormones that help it fight disease. But these hormones have side effects which raise blood glucose levels and interfere with the blood glucose lowering effects of insulin. Preparing a plan for sick days with the support of the patient’s doctors, dietitians, and diabetes educations will help manage these types of days easier. Symptoms to be mindful of are: sick or have had a fever for a couple of days vomiting or having diarrhea for more than 6 hours, moderate to large amounts of ketones in your urine , glucose levels are higher than 240 (even with the assistance of extra insulin) The plan of care includes encouraging the patient to: Continue taking insulin Maintain a daily eating regimen Not to skip meals Consume an adequate amount of fluids. Monitor blood sugar levels every 4 hours Test for ketones in urine if blood sugar levels are >240 mg/dl DKA Case Study; Gabrielle Rutenberg 30 This plan should also consider the fact that food may not be appealing at the time of sickness; however, the individual should still be eating the normal number of calories by eating foods that are easy to digest, such as: regular gelatin (not diet), crackers, soups, and applesauce (sweetened). If the foods are intolerable the patient needs to consider hydration as the sole source of nutrition. In this case, fluids that contain a source of carbohydrates and salt are recommended (i.e juices, smoothies, milk, Gatorade). The goal is 50 grams of carbohydrate every three to four hours. (16) Nutritional supplements can also be used such as Glucerna or Sugar-Free Health shakes, which can help provide the individual with adequate calories and protein if oral intake is low. Many medications you take for short-term illnesses can affect your blood glucose levels, even if they don't contain sugar. For example, aspirin in large doses can lower blood glucose levels. Some antibiotics lower blood glucose levels in people with type 2 diabetes who take diabetes pills. Decongestants and some products for treating colds raise blood glucose levels. It is important to stay in contact with your doctor during these times. (16) Presentation of the Patient I.N. is a 55 year old Caucasian female who lives in Belleville, New Jersey. She lives on the second floor of a two-family house. Her past medical history includes hypertension, type 2 diabetes, obesity, schizophrenia, and depression. She had been admitted to Clara Maass Medical Center back in July of 2014 for a psychiatric evaluation due to feelings of paranoia and inability to leave her house because of this. Her brother had found I.N. in her house and brought her to the hospital to be evaluated. 5 days later I.N. was cleared to be discharged. DKA Case Study; Gabrielle Rutenberg 31 On November 6, 2014, I.N. was readmitted to Clara Maass Medical Center, due to slurred speech, nausea/vomiting, and confusion. Her brother had come to visit her at her home where he found I.N. laying on the floor, extremely weak. It was noted that 3 days prior to her admission she had ran out of her insulin and had not obtained more. Her home medications included Metformin, Levemir, Haloperidol, Lisinopril, Benztrophine, Hydrochlorothiazide, and Paliperidon. Medical/Nutritional Hospital Course November 6th: I.N. was brought to the Emergency Department due to slurred speech, nausea/vomiting, and confusion. The medical doctor also observed symptoms of polydipsia and a fever of 101.8 F. The medical team performed a finger sick glucose test, EKG, blood test, and took a stool specimen. The lab results showed: Labs Values WBC Blood glucose 24.8 (high= possible infection/side effect of DKA) 689 mg/Dl (high =hyperglycemia) Bicarbonate 5 mEq/L (low=acidosis) Arterial blood pH 6.8 (low=acidosis) Potassium 5.2 mEq/L (high=acidosis) Sodium 129 mEq/L (low=hyperglycemia) BUN Blood acetone 30 mg/dL (high=compromised kidney function) 1.3 mg/dL (high=compromised kidney function) Positive (acidosis) Serum ketones Positive (ketosis) Creatinine Table.1.5 DKA Case Study; Gabrielle Rutenberg 32 I.N. labs were indicative of DKA. Her EKG showed T-wave inversion in lateral leads. They also took a stool specimen for a WBC, cultures, and C. diff diagnosis. Her white blood cell count was high indicating a possible infection/another side effect of DKA, however she was negative for C. diff. I.N. was started on IV insulin of 10 units, IV fluids (normal saline), and IV insulin drip. November 7th: I.N. was started on a clear liquid diet. She was still receiving IV insulin drips and IV fluids. Her potassium levels fell down to 3 mEq/L due to the insulin therapy, correction of acidosis, and volume expansion decreased. At this time she was put on D5 @ 250 mL/hr with potassium chloride to maintain glucose levels and to increase potassium levels, as well as a K-dur (as potassium supplement). A chest x-ray was also performed to rule out pneumonia or any other respiratory issues, however it came back negative of any abnormalities. Her nutrition initial assessment was performed on this day as well: Initial Assessment Patient name: I.N. Medical Diagnosis: Diabetic ketoacidosis Age: 55 Sex: F Weight: 220 lb. /100 kg Height: 69’’/175 cm BMI: 32 (Grade 1) DKA Case Study; Gabrielle Rutenberg 33 IBW: 160 lb. /73 kg (+10%) Skin: Braden score 13, stage 2 pressure ulcer (sacrum) Current symptoms: nausea, diarrhea, abdominal pain, altered mental status Afebrile: 98.9 F Medications: Insulin sliding scale regular, D5 with 0.45% NaCl + KCL @ 250 mL/hr, K-dur, Pertinent Lab Values: Labs Values Blood glucose 267 mEq/dL (high) Potassium 3.1 mEq/dL (low) Chloride 111 mEq/dL (high) Sodium 140 mEq/dL BUN 31 mg/dL (high) Creatinine 1.1 mg/dL Table 1.6 Food/Nutrition History: Fair po intake prior to admission as per chart. Estimated Calorie Needs: (based on 25-30 kcal/kg of IBW) = 1,825-2,190 kcal/kg Estimated Protein Needs: (based on 1.2 gm/kg of IBW) = 88 gm/kg Estimated Fluid Needs: (based on 25-30 mL/kg of IBW) = 1,825-2,190 kcal/kg Current Diet Order: Clear liquid diet DKA Case Study; Gabrielle Rutenberg 34 PES: Inadequate oral intake related to nausea/abdominal pain as evidenced by <50% of meal consumed. Risk Level: High (follow-up in 3 days) Intervention: When feasible change to high consistent carbohydrate (2000 kcal), 2 gram Na diet. Multivitamin once daily, vitamin C 500 mg BID. Education: Patient felt nauseas and did not want to discuss food at that time. Monitor: Intake, hydration, BS levels, other pertinent labs, skin integrity. Evaluate: Intake/fluids for adequacy, BS control, labs WNL, need for other wound healing nutrients. Goal: Patient able to consume >75% of meals, BS <140, promote wound healing prior to d/c. November 8th: I.N. was given a magnesium supplement due her magnesium levels falling to 1.3 mEq/dL. She is still feeling nauseas. November 9th: I.N. has persistent acidosis. She will continue on IV fluids, D5 @ 200 mL/hr, and is now put on Levemir. She is also NPO for an ultrasound for possible pancreatitis (her lipase levels were in the 3,000’s), however it came back negative. She had also gotten an additional chest x-ray related to her persistent fever, but no abnormalities were seen. November 10th: I.N. started to have diarrhea, however is more alert and oriented. Her IV of D5 is now at 80 mL/hr. She is also still receiving Levemir, and is now on Novolog. However, her fever is still persisting and is at 101.2 F. DKA Case Study; Gabrielle Rutenberg 35 November 11th: Transferred from ICU to 2 North. Bicarbonate levels are up to 19 showing her acidosis is improving. Her temperature is down to 98.4. November 12th: I.N. is transferred back to the ICU due to atrial fibrillation. November 13th: I.N. is transferred to the 6 South (telemetry unit). Her nutrition reassessment is done: Follow up Assessment Medical Diagnosis: Diabetic ketoacidosis Weight: 220 lb. /100 kg Height: 69’’/175 cm BMI: 32 (Grade 1) IBW: 160 lb. /73 kg (+10%) Skin: Braden score 22, pressure ulcer (previously noted as stage 2) Current symptoms: polydipsia, diarrhea, edema (L leg 1+; B/L pedal 1+), po intake >74% Afebrile: none Pertinent Laboratory Values: Labs Values Blood glucose 137 mg/dL (high) Bicarbonate 19 mEq/L (low) Magnesium 1.3 mEq/L (low) Phosphorous 3 mEq/L DKA Case Study; Gabrielle Rutenberg 36 Potassium 4.2 mEq/L Sodium 138 mEq/L BUN 8 mg/dL Creatinine 0.7 mg/dL (low) HbA1c 15.1 % (high) Table 1.7 Medications: Novolog, Levemir, magnesium sulfate Estimated Calorie Needs: (based on 25-30 kcal/kg of IBW) = 1,825-2,190 kcal/kg Estimated Protein Needs: (based on 1.2 gm/kg of IBW) = 88 gm/kg Estimated Fluid Needs: (based on 25-30 mL/kg of IBW) = 1,825-2,190 kcal/kg Current Diet Order: 2 gm Na diet PES: Excessive carbohydrate intake r/t patient not on a diabetic diet as evidenced by BS 137 & HbA1C 15.1%. Risk Level: Moderate (7 day follow-up) Intervention: Change to high consistent carbohydrate (2000 kcal), 2 gram Na diet. Multivitamin once daily, vitamin C 500 mg BID for skin care. Education: Diabetic/low sodium diet. Monitor: Intake, hydration, BS levels, other pertinent labs, skin integrity. Evaluate: Intake for compliance, adequacy of fluids, BS control, labs WNL, need for other wound healing nutrients. DKA Case Study; Gabrielle Rutenberg 37 Goal: Patient will be able to name 2 high carbohydrate foods, 2 sodium substitutes, BS <140, promote wound healing prior to d/c. November 14th: I.N. is discharged to Alaris Health at Belgrove post-acute care center for 1 week. Critical Comments I feel the situation in which I.N. developed DKA was avoidable. During her past admission in July, the medical team and her brother should have noticed that with her history of mental illness she was unfit to live on her own, especially with insulin dependent type 2 diabetes. However, during her admission in November, both the doctors and nurses have followed the correct protocol in diagnosing and treating her DKA. Seeing I.N. between my initial and followup assessment was quite a difference. During our initial assessment she was extremely lethargic and nauseated making it very hard to interact and educate her. During our reassessment I was able to speak with I.N. more and educated her on the basics of diabetes and low sodium diet. Given her history of mental illness I couldn’t go into too much detail, but made sure to explain to her about carbohydrate consistency/not to skip meals, take her insulin regularly even if she is feeling unwell, high carbohydrate foods she should try to avoid, and substitutes to adding salt to meal. However, she seemed a bit distracted and was constantly drinking to quench her thirst. The fact that she was not put on a diabetic diet was a poor decision on doctor’s judgment. Both on the assessment and reassessment, I made sure to put in my recommendation for I.N., however it was never taken. This showed poor teamwork between the dietician and the medical doctor. If I.N. had stayed longer in the hospital I could have reinforced the education with her, since I was unsure how much she was able to comprehend. This is where I feel an outpatient DKA Case Study; Gabrielle Rutenberg 38 service would be more beneficial in patient education, so that the individual can meet with a dietician in a less distracting environment to better understand the disease, its implications, and way to avoid/monitor it. Having social services send her to a post-acute care setting after her discharges was positive idea. Hopefully her family sets her up at some sort of assisted living facility or to live with family members due to the possible severity of her mental illness and ability to monitor her health. Summary Diabetic Ketoacidosis is a serious complication of diabetes. When your body is unable to produce insulin sugar levels rise causing hyperglycemia. Your cells are also unable to utilize the glucose they need for energy, so your body begins to burn fat. This creates ketones, acids that build up in the blood and urine. In high levels, ketones are poisonous, which make the individual extremely sick. Managing your diabetes is a way to prevent DKA, which is why educating the patient is a way of prevention. I.N was readmitted to the hospital November 19th, just 5 days after her discharge. However, this time it was for hypoglycemia. When I had performed her initial assessment, she did not have symptoms of nausea and had a po intake of >75%. This time the doctors had put her on a high consistent carbohydrate diet, 2 gm Na diet. Our intervention included more education, however her cognitive deficits due to her mental illness and lack of desire/motivation were barriers to learning. She definitely lacked the ability to be able to control her diabetes without supervision. She was discharged to West Hudson, a post-acute care setting for a week. Her brother is also looking to figure out a different living situation for her where she can be supervised, which I agree is the right decision for her well-being. DKA Case Study; Gabrielle Rutenberg 39 Medication Bibliography Medication Humalog (Lispro) Novolog (Aspart) Apidra (Glulisine) Humalin R (Novalin) Velosulin NPH Indication Side Effects Rapid-acting insulin to lower blood sugar. Covers insulin needs for meals eaten at the same time as the injection. Hypoglycemia Hypokalemia Short-acting insulin covers insulin needs for meals eaten within 30-60 minutes. Intermediate-acting insulin covers insulin needs for about half the day or overnight. It is used to control the blood sugar overnight, while fasting and between meals. Long-acting insulin covers insulin needs for about one full day. It is used to control the blood sugar overnight, while fasting and between meals. Antidiabetic medicine that decreases the amount of sugar in the blood of people with type 2 diabetes. Hypoglycemia Hypokalemia Hypoglycemia Hypoglycemia Seizures Abdominal pain Hypokalemia Lactic acidosis Increased heart rate Confusion Nausea/vomiting Diarrhea Nausea/vomiting Diarrhea Dry mouth Nervousness Trouble urinating Movement disorders Dizziness Headache Lantus (Glargine) Levemir (Detemir) Metformin (Glucophage) Haloperidol (Haldol) This drug works in the brain to treat schizophrenia. It is also known as a first generation antipsychotic (FGA) or typical antipsychotic. It rebalances dopamine to improve thinking, mood, and behavior. Lisinopril ACE inhibitors that prevent your body from creating a DKA Case Study; Gabrielle Rutenberg 40 (Zestril) hormone known as angiotensin II. They do this by blocking a chemical called angiotensin-converting enzyme. This relaxes your blood vessels and helps to reduce the amount of water put back into your blood by the kidneys Treats involuntary movements due to the side effects of certain psychiatric drugs such as haloperidol. Extreme tiredness Nausea Diarrhea Weakness Drowsiness. Dry mouth. Difficulty urinating. Constipation. Benztropine (Cogentin) Hydrochlorothiazide (Microzide) Treats high blood pressure and fluid retention (edema). This medicine is a diuretic (water pill). Upset stomach Dizziness Headache Paliperidon (Invega) Stiff/restless muscles Fever Sweating Confusion Fast heartbeat Tremors K-dur Diarrhea Gas Nausea Stomach discomfort Vomiting Dextrose 5% solution It works in the brain to treat schizophrenia and other schizoaffective disorders. This drug rebalances dopamine and serotonin to improve thinking, mood, and behavior. Used to prevent or to treat low blood levels of potassium (hypokalemia). Potassium levels can be low as a result of a disease or from taking certain medicines, or after a prolonged illness with diarrhea or vomiting. Dextrose 5% in water is injected into a vein through an IV to replace lost fluids and provide carbohydrates to the body. It is used hypoglycemia, insulin shock, or dehydration. Dextrose 5% in water is also given for nutritional support to patients who are unable to eat because of illness, injury, or other medical condition. Hyperglycemia Hyperosmolarity Infection at injection site Increase fluid volumes DKA Case Study; Gabrielle Rutenberg 41 DKA Case Study; Gabrielle Rutenberg 42