File
advertisement
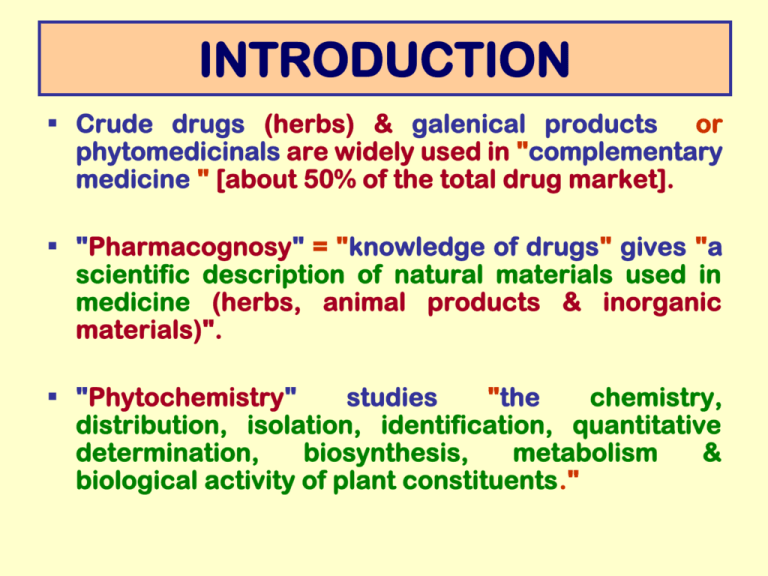
INTRODUCTION Crude drugs (herbs) & galenical products or phytomedicinals are widely used in "complementary medicine " [about 50% of the total drug market]. "Pharmacognosy" = "knowledge of drugs" gives "a scientific description of natural materials used in medicine (herbs, animal products & inorganic materials)". "Phytochemistry" studies "the chemistry, distribution, isolation, identification, quantitative determination, biosynthesis, metabolism & biological activity of plant constituents." Role of natural products in modern medicine Useful drugs which cannot be commercially produced by synthesis e.g. opium, ergot & cinchona alkaloids, digitalis glycosides & most antibiotics. Basic compounds, which could be modified to be more effective or less toxic e.g. morphine molecule. Models for production of synthetic analogues with similar physiological activities, e.g. procaine. Starting materials for production of potent drugs e.g. hydrocortisone & steroidal hormones from stigmasterol & saponins. Different forms of plant products Different forms are supplied based on: ♣ Aim of use ♣ Nature of active ingredients ♣ Economic factors 1. Fresh plant materials [especially in perfume industry]. 2. Dried plant materials: [flavoring agents, spices & drugs where dosage is not critical]. 3. Acellular products : materials derived directly from plants [gums, resins, & fixed & volatile oils]. 4. Galenical preparations: [plant extracts & tinctures] 5. Processed extracts : standardized to contain a certain concentration of the active principle. 6. Pure compounds : most required in pharmaceutical formulations as they facilitate proper standardization of biological activity & quality control Primary & secondary plant metabolites Plant metabolites : organic substances formed & accumulated by plants. 1. Primary metabolites : essential for life & present in all organisms e.g. carbohydrates, proteins, fats, & nucleic acids 2. Secondary metabolites: formed as a defense against predators, attractants (volatile or colored) or detoxifying agents. Mostly pharmacologically active & found in specific organisms or group of organisms. Some metabolites could be included in both divisions e.g. certain fatty acids & sugars. Nomenclature of plant constituents Systematic nomenclature is difficult due to complexity of structure. Naming is based on trivial nomenclature. Root names are derived from: 1. Name of the discoverer e.g. Pelletierine alkaloid after Pelletier 2. Common name of the plant e.g. vinca alkaloids, (vincristine & vinblastine), ergot alkaloids (ergometrine & ergotamine) 3. Latin name of the plant e.g. visnagin from Ammi visnaga & digitoxin from Digitalis lanata 4. Biological action e.g. emetine alkaloid which produces emesis Classification of plant constituents Plant constituents occur as: Single chemicals e.g. glycosides & alkaloids …….. Mixtures of compounds e.g. gums, fixed oils, fats, waxes, volatile oils, resins & resin combinations. Classification of plant constituents may be according to: A. Pharmacological activity [analgesics, laxatives, cardiotonics etc……..] B. Biosynthetic origin, solubility properties & key functional groups C. Chemistry properties & common physical B.According to biosynthetic origin, solubility properties & key functional groups: 1. 2. 3. 4. 5. 6. Phenolics: e.g. flavonoids & their glycosides, phenyl propanoids, anthocyanins, xanthones, tannins & quinones Terpenoids: e.g. carotenoids, steroids & the major constituents of volatile oils Organic acids & lipids: e.g. simple organic acids (citric, oxalic & ascorbic), fatty acids (in the form of esters in fixed oils, fats & waxes) Nitrogenous compounds: e.g. alkaloids & cyanogenic glycosides Water-soluble carbohydrates & their derivatives: e.g. monosaccharides, oligosaccharides & watersoluble glycosides Macromolecules: e.g. proteins & polysaccharides C.According to chemistry & common physical properties This classification will be adopted convenience, the major groups are: for 1.Volatile oils, Resins & Resin combinations 2.Carbohydrates 3.Bitter Principles 4.Tannins 5.Alkaloids 6.Glycosides VOLATILE, ETHEREAL or ESSENTIAL OILS "Volatile" or "ethereal": as they easily evaporate on exposure to air at room temperature (volatile, from the Latin "volare" i.e. to fly & ethereal = ether-like in their volatility) "Essential": as they mostly represent the "essences" or principal active principles of the plants in which they occur. They differ entirely from "fixed oils“ in both chemical & physical properties. Major Differences between volatile & fixed oils Property Volatile oil Fixed oil Volatilization at ordinary temperature Volatile Non-volatile Solubility Soluble in organic solvents (ether, CHCl3) & alcohol Limited solubility in organic solvents, almost insoluble in alcohol Stain on filter paper Transient Permanent & greasy Composition Complex mixtures of hydrocarbons & oxygenated compounds Triglycerides of fatty acids e.g. palmitic, stearic, oleic…….. Response to long exposure to air & light (oxidation) Resinification Rancidity Saponification with caustic alkali (KOH) Negative Positive Historical In ancient Egypt: embalming process (antibacterial properties of essential oils & resins). In the Roman culture: aromatic essences in massage & baths. Incenses: [in temples, churches & mosques] consist of resins rich in volatile oils In folk medicine: inhalation of aromatics as tranquilizers (e.g. incenses in case of irritability) or stimulants (e.g. onions in case of fainting) Distribution & Occurrence Animal sources: Botanical sources: Musk, musk-like products (civet, castoreum) & ambergris Mainly in higher plants Secretions produced for attraction or protection Especially in Pinaceae, Lauraceae, Rutaceae, Myrtaceae, Labiateae, Zingiberaceae, Umbellifereae, & Compositeae. Free & Combined Forms of Volatile Oils They may be present : 1. Free aromatic characteristic odor, or 2. Combined with: Sugars glycosides Gums, resins or both oleo-gums, oleoresins or oleo-gum-resins. Location in the plant They may be: Diffused in all plant tissues (e.g. Pinaceae, Conifers) Accumulated in specialized secretory structures usually on or near the surface of the plant e.g.: 1. Modified parenchyma or oil (Lauraceae & Zingiberaceae) cells: 2. Glandular hairs: (Labiateae) 3. Oil tubes or Vittae: (Umbellifereae) 4. Oil glands: (Rutaceae & Pinaceae) Distribution in plant organs V. O. may accumulate in all types of plant organs: Flowers e.g. rose Leaves e.g. eucalyptus & peppermint Barks e.g. cinnamon Woods e.g. sandalwood Roots e.g. vetiver Rhizomes e.g. ginger Fruits e.g. umbelliferous & citrus Seeds e.g. cardamon Variation in composition of v. oils from different organs of the same plant 1. Cinnamon tree: bark oil rich in cinnamaldehyde leaf oil rich in eugenol root oil rich in camphor 2. Bitter orange tree: "Bitter orange oil": from the fresh pericarp of the fruit (rind or zest), "Neroli oil": from the flowers "Petit grain oil": from the leaves, twigs & unripe fruits. These oils are different in composition & aroma Physiological role of V.O. in the plant 1. Waste products of metabolism (detoxifying agents) 2. Energy producers in case of deficiency from CO2 assimilation 3. H+ donors in certain metabolic reactions 4. Protectants against predators: e.g. insect repellents & antifungals (i.e. for defense). 5. Pollinators: attracting insects during crosspollination (due to their nice odors). Common Physical Characters 1. Colorless, pleasant smelling liquids, volatile at room temperature 2. Steam distillable 3. High refractive index 4. Mostly optically active 5. Density < water (i.e. lighter than water) except for few ones 6. Immiscible with water, but sufficiently soluble to impart a fragrance to water aromatic waters [hydrosols] 7. Soluble in alcohol & common organic solvents 8. Darken in color if exposed to air & light (resinification) Exceptions 1. Oils of cinnamon, clove & winter green are heavier than water 2. Oils of anise & rose solidify just below room temperature (15 & 18oC, respectively) 3. Oils containing azulenes are colored (e.g. oil of chamomile is blue). Chemical Composition V. O. are complex mixtures of hydrocarbons & oxygenated compounds [alcohols, phenols, ethers, aldehydes, ketones, oxides, peroxides & esters]. All of these contribute to the odor & physiological activity of the oil. Few oils consist of one main component e.g. 1. Oil of mustard (93% allylisothiocyanate) 2. Oil of clove (85% eugenol) Most V. O. constituents belong to 2 main groups: 1. Terpenoids [derived from acetate] & 2. Phenylpropanoids [aromatic derived from phenylpropane] compounds, Variation in Physico-Chemical Characteristics Most important influencing factors are: The environmental conditions under which the plant is grown The method used for preparation of the oil Medicinal & Commercial Uses of V.O. 1. Therapeutic & medicinal uses: local stimulants, carminatives, diuretics, mild antiseptics, local irritants, anthelmintics, parasiticides … 2. Spices & condiments: in food seasoning (to impart aroma & flavor) or as preservatives 3. Flavoring agents: in food (e.g. beverages, soups, bakery products, confectionery) & pharmaceutical industries 4. Aromatic agents: in all types of perfume industries (cosmetics, soaps, deodorizers, household cleaners, polishes & insecticides) Methods of Preparation of Volatile Oils Distillation Scarification & Expression Water Distillation Sponge Method Steam Distillation Ecuelle a piquer Method Water & Steam Distillation Direct Steam Distillation Extraction Enzymatic Hydrolysis Extraction with Volatile Solvents Extraction with Non-Volatile Solvents Expression of Rasping Process Enfleurage Method Machine Processes Pneumatic Method Maceration Method Selection of the suitable method is done according to : 1. The condition of the plant material (moisture content, degree of comminution) 2. The localization of the oil in the plant (superficial or deep) 3. The amount of the oil 4. The nature of the oil constituents Distillation methods Principle Most volatile oil constituents boil between 150300ºC. In order to reduce decomposition, volatile oils are distilled in the presence of water. The mixture will boil below 100ºC [Dalton’s law of partial pressure : “When 2 immiscible liquids are heated together, they will boil at a temperature below the boiling point of either one”]. The oil is carried over with steam in the form of vapor Distillation methods Application: preparation of thermostable oils, present in large amounts & not rich in esters (e.g. oils of turpentine, peppermint, cardamon, anise, eucalyptus) Types of distillation: 1. Water-distillation 2. Steam distillation Water-and-steam distillation Direct-steam distillation Distillation: Terminology Hydrodiffusion = process by which water or steam penetrates the plant tissues to take over the oil Aromatic water = Hydrosols = distilled aqueous layer saturated with oil e.g. rose, orange flower & peppermint waters Cohobation = return of aromatic water to the distillation chamber, in water distillation, in order to recover the dissolved oil. Distillation methods Steam Distillation H2O Distillation H2O & Steam Direct Steam Plant material Dried & fresh (petals), not injured by boiling with H2O Dried & fresh, injured by direct boiling with H2O Fresh ( i.e. moisture) Commercial preparations Oils of turpentine & rose Oils of clove, cinnamon & citronella Oil of peppermint -H2O present but not in contact with the plant. -Steam is generated in the still & penetrates the drug -Dried material is moistened before charging -H2O is absent. Mode of charging Plant material dipped in H2O Steam pressure Temperature containing -Steam is introduced by pipes & forced through the plant material placed on perforated trays atmospheric Can be modified 100ºC Can be modified Rate & yield Relatively low Better The best Advantages -Least expensive Hydrolysis is reduced -Cohobation is allowed Method suitable for oils rich in esters & high b. p. constituents -Esters are hydrolyzed. -H2O sol. & high b.p. constituents are not distilled -Not suitable for powders, efficient if material entire or crushed -Hydrodiffusion may be reduced due to lumping or channeling Disadvantages Distillation apparatus Consists of 3 parts: 1. The distillation chamber made of stainless steel free from any Fe+++ ions to avoid degradation of the oil constituents darker oils. 2. The condensing system 3. The receiver e.g. Florentine receivers which allow separation of the oily layer from water in the distillate (oils lighter or heavier than water) Florentine Receivers Purification (Rectification) of distilled oils Bad smelling or dark colored oils are purified by: 1. Redistillation or dry distillation under reduced pressure 2. Dehydration by passing over anhydrous sodium sulphate Remarks 1. Distillation should be done just after comminution [ i.e. reduction in size, crushing, powdering) prevent loss by evaporation or deterioration of the oil. 2. Coarse comminution increase "Hydrodiffusion" oils with better yield & quality. 3. High temperature & water distilled oils differing in composition from natural oils [artifacts]. 4. Insufficient distillation time (shorter) fractionation of the oil. 5. Hydrolytic products (e.g. lower alcohols & acids) are watersoluble & remain in the distillation chamber. 6. Steam volatile impurities e.g. amines & furfural (degradation product of carbohydrates) contaminate the final product. 7. Sensitive constituents could be affected by boiling water e.g. Esters hydrolyzed. Tertiary alcohols dehydrated hydrocarbons. Unsaturated hydrocarbons polymerized. Scarification & Expression Methods Principle Mechanical procedures carried at room temperature & based on puncturing & squeezing of the plant material to liberate the oil, which is collected. Applications Preparation of heat sensitive oils, present in large amounts in outer peels of fruits e.g. Citrus fruits (Rutaceae) as orange, lemon & bergamot. Scarification & Expression Methods The peel of Citrus fruits consists of 2 distinct layers: 1. Outer colored zone (waxes + pigments + oil glands) 2. Inner white zone (pectin + cellulose). Scarification & Expression Methods The process involves 3 steps: 1. Squeezing of the peel under a stream of water emulsion (volatile oil + water + pectin + cellulose + pigments + traces of waxes). 2. Centrifugation (to remove water + pectin + cellulose) 3. Strong cooling (to remove waxes) Scarification & Expression Methods A- Sponge Method Based on squeezing the removed peels e.g. orange 1. Fruits washed, cut into halves & fleshy parts removed. 2. Peels soaked in water, turned inside out then pressed between a convex projection & a sponge. 3. Sponge (saturated with oil emulsion) periodically squeezed in a vessel The tissue of the sponge serves for: 1. Collection of the oil 2. Filtration of the product from any particles of the inner white zone of the peel. Scarification & Expression Methods B- Ecuelle-à-piquer method Based on puncturing (scarifying) the surface of whole fruits (lemon), the oil exudes from the outer zone of the peels in the form of emulsion. The instrument is funnel-shaped, formed of a shallow bowl with a tubular projection at the center. The bowl bears numerous pins which scarify the oil glands to release the oil. The tubular part serves as: 1. Handle to rotate the instrument. 2. Receiver to collect the oil. Scarification & Expression Methods C- Expression of rasping process Based on removal of the outer layer of the peel with a grater, collecting the rasping in special bags then strong pressing. The oil emulsion is collected in large vessels D- Machine processes Based on the same principles as the above 3 traditional methods A, B & C but carried out by machines. Solvent extraction methods Principle Based on extraction of the volatile oil from the plant material with a suitable solvent According to the nature of the solvent used, three types are distinguished: 1. Volatile solvent extraction 2. Non-volatile solvent extraction 3. Supercritical fluid extraction Solvent extraction methods-Application Preparation of delicate flower oils e.g. jasmine, violet, tuberose & narcissus which are: 1. Present in very small amounts, not easily obtained by distillation or expression 2. Oils formed of thermolabile constituents (i.e. easily decomposed by heat) Volatile solvent-extraction Preparation of "floral concretes" 1. Solvents used: petroleum ether & n-hexane 2. Extraction (“percolation” or “maceration” at room temperature, “continuous hot extraction” in a Soxhlet apparatus at constant temperature) 3. Solvent removal (distillation under reduced pressure) Percolator Soxhlet apparatus Volatile solvent-extraction Floral concrete = Fragrant constituents + Fats + Waxes + Albuminous matter + Fat soluble pigments e.g. "floral concrete" of jasmine is semi-solid & yellowish-orange in color. Floral absolute = consists mostly of the oxygenated constituents of the oil. More expensive & purified than the corresponding concrete. Preparation: repeated extraction with absolute alcohol Impurities: removed by strong cooling & filtration Solvent removal : by distillation. Non-volatile solvent extraction Application: Preparation of natural flower producing the finest perfumes. oils Principle: based on the liposolubility of volatile oils Solvents: Lipids of high degree of purity e.g. Fats (lard : tallow in a mixture 2:1) Fixed (olive oil) Techniques: Enfleurage (hot & cold) Pneumatic method Maceration (in fixed oils) Enfleurage Process- Preparation of jasmine oil Equipment: Great number of glass plates closely arranged in wooden frames (or chassis). Procedure: 1. 2. 3. 4. 5. 6. Spread the mixture of fat (lard / tallow 2: 1) on both surfaces of each glass plate. Cover the top of each plate with flowers or petals, so that each layer of flowers is enclosed between 2 layers of fat. Replace old flowers by fresh ones every 2-3 days Repeat the process until the fat is saturated with the oil Remove the last charge of flowers from the fat ("Defleurage") Scrap & collect the fat layers, warm, filter through gauze & cool “Enfleurage product” or “Floral pomade” Enfleurage Process Flower Petals Add fat mixture [Lard & tallow (2 : 1)] 1) Enfleurage Product (floral pomade) [Fat saturated with oil] * Add absolute alcohol * Triple extraction * Cooling (remove most of fat) 2) Triple extract [alc. solution of vol. oil + pigments + traces of fats] Evaporation of alcohol or fractional distillation 3)Absolute of Enfleurage [Semi-solid, alcohol-free product] Dilution with H2O + NaCl 4)Volatile oil Jasmine flowers “Enfleurage” Process Cold Enfleurage Hot Enfleurage Super critical fluid extraction Principle: based on using liquefied gases e.g. CO2 under specific temperatures & pressures as extracting solvents. Under these conditions these gases are liquids but maintain the penetrating properties of gases & allow more efficient extraction. The oils obtained are of closest composition to the natural oils. Process Distillation Applications For dried & Advantages fresh Cheapest Disadvantages method High temperature material, rich in volatile (apparatus, solvent & presence of water oils with thermostable & source of heat) may constituents constituents. Scarification For preparation of oils -Carried & Expression present in at room Expensive large temperature need amounts in outer peels oils Suitable for -Carried at room or Expensive low temperature sensitive oils present in small amounts to high with more natural odors. material with heat- due of sensitive constituents. fresh the number of workers of fruits & rich in heat- -Yields Extraction affect oils number with workers. more natural odors to use of solvent & / or high -Yields due of Methods based on enzymatic hydrolysis of glycosides Glycosides with volatile aglycones are found in: 1. Volatile oil-containing plants e.g. mint, rosemary, Pinus spp., cinnamon & celery. 2. Plants devoid of volatile oils e.g. Gaultheria spp., black mustard & bitter almond. Black mustard The volatile aglycones are known as the "essential oils" of the plants e.g. 1. Methyl salycilate = oil of wintergreen 2. Allyl isothiocyanate = volatile oil of black mustard 3. Benzaldehyde = volatile oil of bitter almond Bitter almond Gaultheria sp. Methods based on enzymatic hydrolysis of glycosides: Principle 1. Plant material + enzymatic hydrolysis volatile aglycones in the hydrolysate 2. Hydrolysate + distillation or extraction with organic solvent volatile aglycone Fixed oil if present in large amount in the plant material should be removed by expression before hydrolysis Examples of glycosides with volatile aglycones Plant name Gaultheria procumbens (Ericaceae) & Betula lenta Non-volatile Glycoside Volatile aglycone Other hydrolytic products Hydrolytic enzyme Gaultherin Methyl salicylate Primeverose (Xylose + Glucose) Gaultherase Monotropin Methyl salicylate Glucose Gein Eugenol Glucose -Glucosidase Sinigrin Allyl isothiocyanate Glucose + Potassium acid sulfate Myrosin Glucovanillin Vanillin Glucose -Glucosidase Amygdalin Benzaldelhyde Gentiobiose (2 glucose units) + HCN Amygdalase & Emulsin (Betulaceae) Geum urbanum (Rosaceae) Brassica nigra (Brassicaceae) Vanilla planifolia (Orchidaceae) Amygdala amara (Prunus amygdalus, Rosaceae) Preparation & purification of volatile oil of bitter almond 1. 2. 3. Seeds crushed & fixed oil removed by expression Cake macerated in water for few hours, at 40ºC in a closed vessel Amygdalin hydrolysis (Amygdalase + Emulsin) Benzaldelhyde + 2 glucose + HCN Steam distillation benzaldehyde + HCN (free state or as benzaldehyde cyanohydrin) Purification of bitter almond oil or "Removal or fixation of HCN" By transformation to the non-volatile Ca2Fe(CN) 6 : Impure distilled oil + Ca (OH)2 +FeSO4+ redistillation Detection of residual HCN in the purified oil Prussian blue test: Oil + NaOH (t.s.) & shake, if any traces of HCN NaCN + FeSO4 (traces of Fe+++ ions) + HCl & warm Fe4 Fe (CN)6]3 (ferric ferro cyanide), bluish black in color. 2 HCN + Ca (OH) 2 Volatile 3 Ca (CN) 2 + FeSO4 Ca (CN)2 + 2H2O Non-volatile Ca2 Fe (CN)6 + CaSO4 Non-volatile Determination of percentage of volatile oil in plant material Miscibility with alcohol 1. Most volatile oils are miscible with absolute alcohol. 2. Oils highly miscible with alcohol of low concentrations are usually rich in oxygenated constituents. 3. Decreased miscibility with alcohol of low concentrations adulteration with non-polar solvents e.g. petroleum ether (turbidity) or fixed oils % v/w = Vol of oil × 100 / Wt of drug Physical Examination: helps in evaluation of the oil sample & detection of adulterants Odor Detection of any abnormal odor (by smelling 1 or 2 drops of the oil applied on a filter paper) adulteration or deterioration during storage e.g. orange oil acquires a caraway odor on bad storage due to autoxidation of limonene to carvone & carveol Solubility 1. Oils are soluble in non-polar solvents as benzene, carbon disulfide & light petroleum. 2. Any turbidity moisture Specific gravity Apparatus: pycnometer (specific gravity bottle) Sp. gr. gives an indication on composition 1. Oils with sp. gr. < 0.9, rich in hydrocarbons & aliphatic compounds 2. Oils with sp. gr. > 1.0, rich in aromatic & S compounds. 3. Oils with 0.9 > sp. gr. < 1.0, contain different types of constituents Optical rotation [Apparatus: Polarimeter ] 1. Determination helps in detection of adulteration & identification of the variety of the sample e.g. French oil of turpentine is levorotatory [l (-)] as it contains l-pinene in high concentration. American oil of turpentine is dextroratory [d (+)] as its major constituent is d-pinene. 2. Gives indication on the method of preparation of the volatile oil isolate: All synthetic compounds are racemic (dl). Natural compounds are generally optically active present in (l) or (d) forms. Example: natural camphor is (l) or (d) while synthetic camphor is (dl). Refractive index [Apparatus: Refractometer] Refractive Indices of volatile oils range from 1.4- 1.6 any deviation adulteration Pycnometer Polarimeter Abbe refractometer Chemistry of volatile oils constituents Types of constituents detected in volatile oils: V. O. are complex mixtures formed of: 1. Terpenoids (mainly mono- & sesquiterpenoids) 2. Phenyl propanoids (C6-C3, aromatic) 3. Aliphatic compounds (acyclic, straight chain compounds which may be terpenoids). 4. Miscellaneous compounds organo-sulfur compounds. e.g. organo-nitrogen & Each group includes non-oxygenated (hydrocarbons) & oxygenated compounds. Oxygenated constituents are generally responsible for the characteristic odor of the oil. Removal of terpenoid hydrocarbons Oils rich in terpenoid hydrocarbons deteriorate rapidly on storage due to oxidation & polymerization bad smelling (with turpentine-like odor) & resinified products. Removal of most terpenoid hydrocarbons "terpenelessoils" by any of the following methods: 1. Fractional distillation under reduced pressure: hydrocarbons have lower b.p. than oxygenated compounds, they distill first & are rejected. 2. Column chromatography on silica gel: hydrocarbons are eluted with n-hexane then oxygenated compounds with absolute alcohol. 3. Selective extraction oxygenated components with dilute alcohol followed by distillation. “Terpeneless oils” Oils from which most terpene hydrocarbons are removed 1. More expensive than natural oils 2. Richer in compounds. 3. More soluble in low-strength alcohols. 4. Used in smaller amounts to give the same strength of odor. 5. oxygenated More stable being less liable to deterioration “Volatile oil isolates” An isolate is a single chemical substance isolated from the oil “Oleoptene & Stearoptene” 1. Stearoptene = solid fraction separating on cooling a v.o. (previously known as camphors), consists of 1 or more solid (mainly oxygenated ) compounds 2. Oleoptene = remaining liquid fraction, mainly formed of hydrocarbons Isolation of volatile oil constituents Physical methods (cooling, fractional distillation, fractional crystallization & preparative gas chromatography) Chemical methods depend on: 1. Solubility differences in acids or alkalis 2. Derivatization (due to presence of functional groups). 3. Adduct formation compounds). (specific for certain Chemical methods for isolation of V.O. constituents Solubility in alkalis: 1. Compounds containing -COOH group (strongly acidic) + mild alkali (Na2CO3) water soluble Na salts (decomposed by acids). 2. Phenolic compounds (mild acids) + aqueous NaOH or KOH (strong alkalis) water soluble Na or K phenates (decomposed by acids) phenol. Derivatization: 1. Alcohols esterification phenyl urethans or acid phthalates 2. Carbonyl compounds derivatives e.g. crystalline semicarbazones, phenyl hydrazones & oximes. bisulfites, Formation of crystalline additive products 1. Geraniol, benzyl & cinnamyl alcohols + anhydrous CaCl2. 2. Carvone + H2S gas in presence of NH3. 3. Cineole + strong acids (e.g. H3PO4) & resorcinol 4. Unsaturated terpene hydrocarbons + HCl, HBr & NOCl (nitrosyl chloride or Tilden’s reagent). 5. Azulenes + Strong mineral acids e.g. H3PO4 and H2SO4 Ferrocyannic acid Nitrocompounds e.g. picric, styphnic & tortylic acids. Terpenoids (Terpenes) They constitute the largest known group of secondary metabolites. The term “terpenes” should better be used to indicate the unsaturated hydrocarbons All yield isoprene as final product of destructive distillation (= pyrolysis). Isoprene, a 5 carbon-atom unit, is the building unit of all terpenoids 1 CH 2 4 H2C 3 C h t C 2 Abbreviated structure h=head, t=tail CH3 Isoprene, 2-methyl 1:3 butadiene, 1,3 isopentene Isoprene rule for formation of terpenoids Theoretical biogenetic rule which states that: “Each group of terpenes originates from the head-to-tail condensation of a variable number of isoprene units”. Isoprene Isoprene Isoprene Isoprene Monocyclic Acyclic monoterpene monoterpene Bicyclic Monoterpene Isoprene Acyclic sesquiterpene Monocyclic sesquiterpene Coupling of isoprene units to yield mono- & sesquiterpenoids Terpenoids in Essential Oils Thousands are identified in essential oils Mainly mono- or sesquiterpenoids (volatile & of low molecular weight) Acyclic (i.e. aliphatic) or Alicyclic (i.e. with nonaromatic ring-structures) Hydrocarbons or oxygenated (alcohols, aldehydes, ketones, esters, ethers, oxides or peroxides) Often optically active occurring as d-, l- & dl isomers Monoterpenoids (C 10) 1. Most abundant class of essential oil constituents. 2. Consist of 2 molecules of isoprene 3. Hydrocarbons have the empirical formula C10H16 4. Acyclic or alicyclic (mainly mono- & bicyclic) Terpenoids in Essential Oils Sesquiterpenoids (C15) 1. Present in the high boiling point fractions of the oils (250-280oC). 2. Mostly viscous liquids or may be crystalline. 3. Consist of 3 molecules of isoprene 4. Hydrocarbons have the empirical formula C15H24 5. Acyclic, monocyclic or polycyclic. 6. Occur in more than 100 different skeletons with ring size ranging from 4, 7, 8, 10 & 11 C atoms. Azulenes (C15H18) 1. Usually discussed under sesquiterpenoids because they have the same number of C atoms & distill in the same boiling range. 2. But, they possess aromatic properties due to high conjugation & are highly colored (generally blue, green or violet) e.g. Chamazulene in oil of chamomile. Nomenclature of terpenoids Chemical names are derived from the corresponding saturated hydrocarbon skeleton 1. 2. The acyclic monoterpenoids are 2, 6-dimethyl octane (myrcane) derivatives. Most monocyclic monoterpenoids are para-menthane rarely metamenthane derivatives. 3. Bicyclic monoterpenoids are thujane, carane, pinane, camphane or fenchane derivatives. 4. Acyclic sesquiterpenoids are trimethyldodecane derivatives. 5. Mono- & polycyclic sesquiterpenoids are bisabolane, humulane, elemane, germacrane cadinane, santalane, cedrane derivatives etc… 6. The number & position of the double bonds are indicated e.g. a double bond between C1 & C2 by 1 ; while a double bond between C1 & C6 by 1(6) Trivial names are better adopted for facility. Saturated hydrocarbon skeletons of mono- & sesquiterpenoids MONOTERPENOIDS SESQUITERPENOIDS Myrcane p-Menthane m-Menthane Trimethyldodecane Thujane Carane Elemane Pinane Germacrane Bornane (Camphane) Fenchane Bisabolane iso-Camphane Humulane Cadinane Isomerism of Monoterpenoids Structural isomerism due to shift in the double bonds, e.g. Myrcene Ocimene Limonene Terpinene Structural isomerism due to shift in the position of a substituent group e.g. pmenthadiene & mmenthadiene derivatives. Limonene Sylvestrene Isomerism of Monoterpenoids Geometrical isomerism: e.g. the cis-trans isomeric alcohols, nerol & geraniol. CH 2OH H Geraniol H CH 2OH Nerol Optical isomerism: due to the presence of one or more asymmetric C atoms e.g. dipentene occurs in d, l & dl forms due to asymmetry at C4 (not involved in a double bond), while terpinene is optically inactive. Strainless ring isomerism: chair & boat configurations more stable than planar configuration. Isomerism due to molecular rearrangement of the ring structures: e.g. from pinane to camphane etc…. Phenyl propanoids (C6-C3) or Aromatic constituents Less common than terpenoids. Contain a C6 phenyl ring to which is attached a C3 propane side chain Many are phenols (e.g. eugenol), phenol ethers (e.g., anethole, safrole, cinnamaldehyde). apiole) or aldehydes (e.g., The propane side chain may be formed of 2 C (C6-C2) or 1 C (C6-C1) e.g. vanillin, methyl salicylate & methyl anthranilate. Certain aromatic C10 compounds e.g. p-cymene, thymol & carvacrol can be described under monoterpenoids. Examples of phenyl propanoids in volatile oils OH OH p-Cymene OCH3 OH Thymol Carvacrol O OCH3 O O H CO 3 O OCH 3 Anethole Eugenol Saffrole OH OCH3 Apiole O CHO OCH 3 CHO Vanillin CH2 OH OH Cinnamaldehyde Methyl salycilate Phenyl ethyl alcohol






