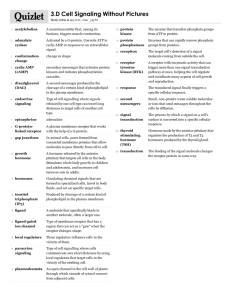
Unit 1 PPT 6 (2cii Signal transduction)
advertisement
AH Biology: Unit 1 Cells and Proteins Membrane Proteins: Signal Transduction Signal transduction • Where extracellular messages are hydrophilic, eg protein hormones such as STH (human growth hormone) and insulin or neurotransmitters such as acetylcholine, they are unable to pass directly through the lipid bilayer. • They are not directly transported across membranes, but instead their message is transduced, ie converted into an intracellular signal by the act of binding to target receptors in the plasma membrane. • We are going to consider, using case studies, three such systems at the cell surface: – – – ion-channel-coupled receptors, eg ligand-gated ion channels G-protein-coupled receptors enzyme-coupled receptors. Ion channel coupling • The next slide illustrates how an action potential travelling along one nerve cell can be transmitted to another. • The junctions between nerves or nerves and muscles are called synapses. • The transduction of the message is required. • The action potential initiates the fusion of neurotransmitters containing vesicles with the presynaptic membrane. These chemicals diffuse across the synapse, triggering ion-gated Na+ channels on the post-synaptic membrane, which initiates the generation of an action potential in the target cell. This may result in action potential propagation down the nerve or the triggering of myofibril contraction in neuromuscular junctions. • Neurotransmitters are rapidly reabsorbed after release or broken down. • This class of transduction is regulated by controlling the influx and efflux of ions with associated voltage-gated or ligand-gated channels. Ligand-gated ion channels + Na Na+ Na+ + Na+ Na Initial influx of Na+ depolarises the membrane. This may trigger local voltagegated channels to open and cause a further influx of Na+. Such rapid depolarisation spreads to other Na+ channels, creating an action potential that spreads to the entire plasma membrane. G-protein-coupled signal transduction G-protein coupled • In this system, G-protein-coupled receptors (GPCR) activate a second protein, the Gprotein. • GPCRs have seven transmembrane alpha-helix domains. • The G-protein is a guanine triphosphate (GTP) binding protein and is bound into the intracellular face of the plasma membrane. • On activation by the GPCR, GTP binds to and activates the G-protein complex. • The G-protein then activates a separate target protein. This is usually an enzyme or ion channel. • If the target protein is an enzyme, activation may start a cascade of small intracellular signalling molecules within the cytoplasm, triggering intracellular events. • If the target protein is an ion channel, activation may result in the mediation of plasma membrane permeability. G-protein-coupled signalling G-protein-coupled signalling case study Production of cyclic AMP (cAMP) • Some classes of GCPR stimulate the production or inhibition of cAMP by adenyl cyclase. • This makes cAMP from ATP in the cytoplasm. This molecule is a potent intracellular signal. It should be noted that there are also GCPR-linked systems whose role is to reduce cAMP levels. • Hormones such as adrenaline, glucagon and thyroid-stimulating hormone (TSH) act on their appropriate receptor, triggering G-protein and switching on adenyl cyclase, which is plasma membrane bound, raising cAMP levels. • cAMP is destroyed by cAMP phosphodiesetrase. G-protein-coupled signalling case study • cAMP can activate a second group of enzymes called cAMP-dependant kinases. • Once bound to cAMP these kinases are activated, releasing active catalytic subunits. These can diffuse through the nuclear pores where, for instance, they can phosphorylate gene regulatory proteins called cAMP response element binding proteins (CREBs), which can then stimulate gene transcription. • Raised cAMP can also trigger some of the effects of the following ligands when stimulated through the appropriate GCPR/G-protein activation. – Adrenaline is a hormone that has many different effects depending on what target receptor it stimulates. Many different classes of adrenaline receptor exist and depending on what type the target cell expresses the effect may be very different. For example, myocardial tissue in the heart will increase the rate and contraction of its cells when triggered by adrenaline. Adipose cells (fat cells) are triggered to break down triglycerides and release fatty acids and glycerol into the bloodstream. – TSH acts on thyroid cells, stimulating thyroid hormone synthesis and secretion. – Glucagon stimulates liver cells to break down stored glycogen to glucose. G-protein-coupled signalling case study Cascades • At each step the generation of multiple linked intracellular messages creates interlinked signal systems called cascades, eg molecule 1 stimulates production of molecule 2 etc. • Cascades can greatly magnify the response. Initial binding of one signal molecule to its GPCR can generate many copies of intracellular signal molecules such as cAMP or IP3. G-protein-coupled signalling case study An example of ion channel regulation by G-protein coupled signalling • Acetylchoine, a neurotransmitter, can reduce cardiac muscle activity. • Efflux of K+ balances influx of Na+ into cytoplasm from outside, and Ca+ from intracellular compartments. • This makes it more difficult for the target membrane to depolarise and trigger cell contraction. • These acetylcholine GPCR are different to the ligand-gated channels found at neuromuscular junctions in skeletal muscle and nerve-tonerve synapses. G-protein-coupled signalling case study Regulation of the cytoskeleton by G-protein-coupled signalling • Two subfamilies of G-proteins, G12/G13 , are involved in cytoskeletal remodelling by promoting actin–myosin contraction. • An associated family Gi is involved with actin fibre polymerisation and membrane protrusion. • Two pathways exist. Stimulation of the G1-linked receptor stimulates the Rac pathway via the production of the intracellular signal for phosphatidyl inositol triphosphate (PIP 3), which induces polymerisation of actin filaments and the extrusion of the cell towards the stimulus. • Simultaneously, stimulation of the G12/G13 linked receptor stimulates the Rho pathway, causing actin–myosin contraction. • All the intermediate messengers are short lived so the stimulus is polarised to the surface in contact with the stimulating ligand. • The Rho and Rac pathways appear to be antagonistic so if Rac is stimulated at the front Rho can only be stimulated at the rear. This allows directed movement towards a stimulus with contraction of cytoskeleton behind and extrusion at the front. • The movement of neutrophils towards bacteria has been characterised in this manner. G-protein-coupled signalling case study Olfactory sensitivity • Olfactory receptors in animals are good examples of GPCRs that are sensitive to odours. • The receptors are localised on modified cilia protrusions from the olfactory neurons. • Different classes of olfactory sensors exist for many different molecules. Each is encoded by different genes. What they all have in common is their mode of action. • Each GPCR activates an olfactory G-protein, which in turn stimulates the production of cAMP by adenyl cyclase. • Increased cAMP levels stimulate associated cAMP-gated Na+ channels, resulting in an influx of Na+ ions depolarising the target membrane. If depolarisation is strong enough an action potential is set up that causes the nerve to fire and send the action potential down the axon, where it generates a nerve impulse. These impulses send sensory information about smell to the central nervous system. G-protein-coupled signalling in photoreception • Vertebrate vision is mediated by photoreceptors coupled to G-proteins. • The photoreceptor is coupled to a G-protein that switches off production of GMP cyclase and results in falling cGMP. This closes associated cGMP-gated cation channels and results in reduced neurotransmitter release from associated neurones when the photoreceptor is illuminated. • cGMP-gated cation channels are kept open by high levels of cGMP, inhibiting depolarisation. As cGMP levels fall these channels close. • The postsynaptic neurones lose their inhibition and an action potential is generated along the neurone as depolarisation is promoted. The neurone is said to be excited. A nerve impulse is then sent along sensory nerves to the CNS. • Light detection and its magnification are discussed in detail in a separate presentation. Enzyme-coupled signalling In this form of signal transduction the binding of a ligand stimulates a transmembrane enzyme directly. These molecules span the bilayer, existing singly or as two proteins stimulated by dimers. Ligand binding stimulates an enzyme directly rather than via a G-protein. The cytoplasmic portion containing the catalytic site may have additional domains. Alternatively, a transmembrane receptor in permanent association with an enzyme undergoes a conformational change on ligand binding which results in enzyme activation. Enzyme-coupled signalling case study Receptor tyrosine kinases (RTKs) • Many enzyme-coupled signalling systems fall into these families. • On stimulation by their appropriate ligand they are able to auto-phosphorylate tyrosine amino acids on their cytoplasmic domains through the activation of their catalytic site. • The phosphorylated tyrosines may in turn provide binding sites for intracellular signals, which are then activated themselves by becoming phosphorylated or by undergoing a conformational change through binding to the phosphorylated tyrosine residues. • In turn this may start an intracellular cascade by activated intracellular signals, including the stimulation of some of the same intracellular signals as G-protein-linked systems, eg phopholipase-C-linked cascades. • Once stimulated and the signal transduced, the receptor–ligand complex is internalised by receptor-mediated endocytosis, switching off the response. The recycled receptor minus ligand is then returned to the plasma membrane. Enzyme-coupled signalling case study: insulin – This hormone triggers many intracellular messengers. Binding to its receptor stimulates phosphorylation of its receptor and associated signal molecules, triggering PIP3 production and its associated cascade. This gives rise to expression of glucose transporter Glut 4 on the plasma membrane, increasing intracellular glucose levels. Additionally, glycolysis and fatty acid synthesis are promoted, as is glycogen synthesis. – Diabetes mellitus is a condition associated with impaired insulin function either through insufficient insulin production (type 1/insulin dependant) or insulin resistance related to insensitivity or density of insulin receptors (type 2/non-insulin-dependant, NIDDM). – Some rare mutations of insulin receptors can give rise to conditions such as Donohue syndrome (leprechaunism). In this case the receptors lose their affinity for insulin because of an inherited recessive mutation, giving rise to complete resistance to insulin. This condition is universally fatal, with most sufferers dying within 2 years of birth. – Other mutations have been identified which relate to reduction in insulin sensitivity by mutations retarding the ability of the cell to recycle the receptor back to the plasma membrane. This may provide a partial model for loss of insulin sensitivity in type 2 (NIDDM) diabetes. Enzyme-coupled signalling case study Insulin action Effect of insulin on glucose uptake and metabolism 1. Insulin binds to its receptor, resulting in activation of tyrosine kinase. 2. Activation of intracellular message cascades trigger the following: • glut-4 transporter to the plasma membrane and influx of glucose • glycogen synthesis • glycolysis • fatty acid synthesis. Enzyme-coupled signalling case study Cell growth factors Examples: epidermal growth factor (EGF), fibroblast growth factors (FGFs) • Most cell growth factors stimulate cell proliferation and cell growth through increasing mitosis and DNA activity. • The genes encoding the growth factor and their receptors are mainly protooncogenes as mutation of the same can give rise to oncogenes, which promote uncontrolled cell division. Enzyme-coupled signalling case study Epidermal growth factor • Coded for by a proto-oncogene. • Its receptor EGFR is an RTK which is also coded for by a proto-oncogene. • The diagram shows the multitudinous ways that the stimulated receptor homo dimer can generate intracellular signals and their potential outcomes. • One effect of EGFR stimulation is the transcription and expression of EFGR genes. • Over-expression of EFGR and EGF is linked to cancer and is an expression of oncogenic mutations. Enzyme-coupled signalling case study Fibroblast growth factors • This family of ligands is involved in the control of cell growth and cell differentiation, vascularisation, wound healing and embryo growth. • They act through an associated family of RTKs called fibroblast growth factor receptors (FGFRs) in a similar manner to EGFRs. • One subclass of receptor, FGFR3, has been associated with bone growth as FGFR3 stimulation seems to inhibits the formation of bone from cartilage. • Mutations in FGFR3 have been associated with achondroplasia, the most common form of dwarfism (~1 in 25000 live births world wide), which results from retarded long bone growth. • Achondroplasia is a non-sex-linked dominant condition in which sufferers inherit an allele for mutated FGFR3 that is overly active. Enzyme-coupled signalling case study Molecular medicine • Current advances in molecular biology are focusing on targeting specific RTKs as those related to cell growth factors are often over-expressed in cancer cells. • Drugs and monoclonal antibodies are in development to try and block such receptors. • Herceptin® and Erbitux® are commercial monoclonal antibodies that block two types of EGF receptor (Her-1 and Her-2) that can be over-expressed in some types of breast cancer. • Iressa® is a tyrosine kinase inhibitor that may block the action of the over-expressed EGF receptors on some lung cancer cells. Enzyme-coupled signalling case study JAK-STAT pathways receptors • This is another class of enzyme-coupled signal receptors. • They are receptors that always exist as two identical single-pass transmembrane proteins that exist as homo dimers. Each of their cytoplasmic tails is bound to a janus kinase (JAK) . • Tyrosine residues are phosphorylated on the cytoplasmic domains on ligand binding, as are associated signal transducer activator of transcription (STAT) molecules. • These STAT molecules dissociate to form cytoplasmic dimers, which enter the nucleus and bind to specific DNA sequences in the promoters of genes that begin transcription. • They differ from RTKs in the directness of their response as few intermediary messengers are utilised. • Growth hormone, erythropoietin and interleukins act by these pathways. Enzyme-coupled signalling case study JAK-STAT pathways of growth hormone (STH)