14-15.Acids and Bases_RVW
advertisement

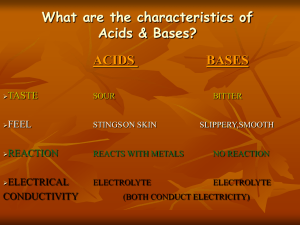
Chemistry Chemistry Name: § 14/15 Test Date: Acids & Bases - REVIEW 1. 2. 3. 4. Compare the Arrhenius and Brønsted-Lowy definitions for acids and bases. What are the properties of acids? of bases? Define amphoteric. Is water amphoteric? Give an example of another amphoteric compond. What are, and give an example, of each: binary acid, polyprotic acid, diprotic acid, and triprotic acid. 5. Define a strong acid/base. Define a weak acid/base. 6. List six strong acids and six strong bases. Strong Acids Strong Bases H + halogen (except HF): HCl alkali metal + OH: LiOH HBr NaOH HI KOH Other heavy alkaline-earth + OH: HClO4 Ca(OH)2 HNO3 Sr(OH)2 H2SO4 Ba(OH)2 7. What acid is in each of the following: A) car batteries, B) Pepsi & Coke, C) blood and seltzer, D) stomach acid, E) vinegar? 8. Is ammonia (NH3) a strong acid, a weak acid, a strong base, or weak base? Explain. 9. Identify the Brønsted-Lowry conjugate acid-base pairs in each of the following reactions: a. hydrochloric acid + water b. carbonic acid + water c. sulfuric acid + water (both reactions) d. phosphoric acid + water (all reactions) 10. Lemon juice has a pH of about 2. Distilled water has a pH of 7. How many times greater is the H3O+ concentration in lemon juice than it is in water? 11. What is the pH, pOH, [H3O+], and [OH–] for the following solutions: a. 2.3 x 10–4 M calcium hydroxide b. 9.4 x 10–3 M sulfuric acid c. A solution made by dissolving 3.00 grams of sodium hydroxide in distilled water and brought up to 250. mL of solution. d. A solution is made by dissolving 20.0 grams of magnesium hydroxide in distilled water and brought up to 1.5 L of solution. 12. A 10.0-mL solution hydrochloric acid of unknown concentration is neutralized by 40.0-mL of a 5.00 x 10–2 M solution of sodium hydroxide. What is the molarity of the hydrochloric acid? Document1 (3/15/2016)