Medical Genetics
advertisement

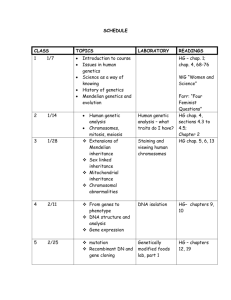
Medical Genetics Mohammed El-Khateeb Dental Postgraduate MG - Lec. 1 3ed July 2013 OBJECTIVES Basic understanding of clinical genetics Be able to draw, and understand, a family tree Have awareness of when you should be considering a genetic condition Have a working knowledge of the most important genetic conditions Know how & when to refer to local specialist genetics services What’s a ___? • Genetics : Is the branch of biology that deals with heredity and variation in all living organisms • The subfields of genetics : Human genetics, Animal genetics, Plant genetics Medical genetics What’s a ___? • Medical Genetics : Is the science or study of biological variation as it pertains to health and disease in human beings. Any application of genetic principles to medical practice. “Genetics – study of individual genes and their effects” Includes studies of inheritance, mapping disease genes, diagnosis, treatment, and genetic counseling History of Medical Genetics • Early Genetics - Biblical, Talmud • Mendel - 1860s • Modern Experimental Genetics - 1900s • Maize, drosophila, mouse • Medical Genetics - 1960s to the present Foundations of Heredity Science Variable traits are inherited Gene – trait-specific unit of heredity Alternative versions of a gene (alleles) determine the trait Each parent transmits an allele to the offspring Gregor Mendel Charles Darwin Mendel studies seven characteristics in the garden pea Mendel deduced the underlying principles of genetics from these patterns 1. Segregation 2. Dominance 3. Independent assortment Alleles: alternative versions of a gene. The gene for a particular inherited character resides at a specific locus (position) on homologous chromosome. For each character, an organism inherits two alleles, one from each parent Medical Genetics: 1960s to the present • DNA Genetics • 1953 - Watson and Crick’s Double Helix • 1992 –2003 Human Genome Project • 2003 -> the future of medical dx & tx • Prenatal Genetics • 1970s - Prenatal Ultrasound & Amniocentesis • Inheritance of Genetically Complex Disorders • – – – • Non-Mendelian Genetics Genomic Imprinting Triple Nucleotide Repeats Mitochondrial Inheritance 1990s - Neuropsychiatric Disorders, Diabetes, Cardiovascular – Interaction of genes with environmental triggers C19th: Mendel discovers basis of inheritance Darwin’s theory of natural selection 1953: Watson and Crick discover structure of DNA 1985: PCR 1986: Duchenne muscular dystrophy gene 1989: Cystic Fibrosis gene 1998: Decision to sequence entire human genome 2001: Human genome sequence completed What is DNA Day? April 1953 Drs. James Watson and Francis Crick determined the structure of DNA (double helix) What is DNA Day? April 1953 April 2003 Drs. James Watson and Francis Crick determined the structure of DNA (double helix) Human Genome Project determined the entire DNA sequence of a human (3 billion letters) What is DNA? • It's a history book - a narrative of the journey of our species through time. • It's a shop manual, with an incredibly detailed blueprint for building every human cell. • And it's a transformative textbook of medicine, with insights that will give health care providers immense new powers to treat, prevent and cure disease." Francis Collins Importance of Genetics to Medicine >12 million Americans with genetic disorders (GD) 80% of MR due to genetic component 2-3% background population risk for a major birth defect (BD) 15% overall miscarriage risk for any pregnancy 25-50% first trimester miscarriage risk 30-50% first trimester losses due to chromosome anomalies >30% pediatric hospital admissions due to GD GD affect all major systems, any age, any race, male or female Importance of Genetics to Medicine Changing focus of medicine: primary care physicians vs specialists prevention vs treatment genetic causation for both rare and common diseases Human Genome Project designer drugs Problem based approach taken in medical schools Genetics as the link between basic research & clinical observation Importance of Genetics to Medicine Triple theme: Genetic traits as they segregate through families allows insights into health of the population Flow of information from DNA to RNA to protein links genetics to physiology Ethical issues linked to treatment, therapy options, research, decision-making and quality of life What are Genetic Variations? • Variations are simply differences in genetic • sequence Variation can be seen at every genetic level: In the DNA In the genes In the chromosomes In the proteins In the function of proteins Classification of genetic disorders • Single gene • Chromosomal • Mitochondrial • Multifactorial • Somatic mutations (cancer) Single Gene Defects Autosomal recessive Autosomal dominant X-linked recessive X-linked dominant Basic Gene Structure Exons Polyadenylation signal Start of transcription Promoter Initiation codon ATG 5’ untranslated region Termination codon Introns UAA UAG UGA 3’ untranslated region Sickle Cell Anemia Inheritance R D X • Single-Gene “Mendelian” Disorders Structural proteins • Osteogenesis imperfecta and Ehlers-Danlos (collagens); Marfan • syndrome (fibrillin); Duchenne and Becker muscular dystrophies (dystrophin) Enzymes and inhibitors • Lysosomal storage diseases; SCID (adenosine deaminase); PKU (phenylalanine hydroxylase); Alpha-1 antitrypsin deficiency • Receptors • Familial hypercholesterolemia (LDL receptor) • Cell growth regulation • Neurofibromatosis type I (neurofibromin); Hereditary • retinoblastoma (Rb) Transporters • Cystic fibrosis (CFTR); Sickle cell disease (Hb); Thalassemias Single gene disorders • Single mutant gene has a large effect • • • on the patient Transmitted in a Mendelian fashion Autosomal dominant, autosomal recessive, X-linked, Y-linked Osteogenesis imperfecta - autosomal dominant • Sickle cell anaemia - autosomal recessive • Haemophilia - X-linked Fertilization: Diploid Genome • • Each parent contributes one genome copy Offspring cells have two near-identical copies Genes & chromosomes Chromosomes • Linear agglomerates of proteins & DNA in the cell’s nucleus • Distributed evenly upon division • Morgan (1910): Genes reside along the chromosomes Mitosis vs. meiosis Meiosis KM 28 Cell Cycle Chromosomes Homologous chromosome: one of a matching pair of chromosomes, one inherited from each parent. Sister chromatids are identical Chromosome Number Constancy in Different Species Buffalo Cat Dog Donkey Goat Horse Human beings Pig Sheep 60 38 78 62 60 64 46 38 54 Genetic Material (chromosomes pairs) Pair of homologous chromosomes Sister chromatids Centromere ISCN 1995 International System for Human Cytogenetic Nomenclature Group A (1-3) Group B (4-5) Group C (6-12, X) Group D (13-15) Group E (16-18) Group F (19-20) Group G (21-22) Chromosomal Rearrangements •Numerical chromosome changes/aneuploidy Result from errors occurring during meiotic or mitotic segregation • Structural chromosome changes Multifactorial inheritance • Familial clustering which does not • • conform to any recognized pattern of Mendelian inheritance Determined by the additive effects of many genes at different loci together with the effect of environment Examples include congenital malformations, asthma, schizophrenia, diabetes , hypertension Etiology of diseases. For any condition the overall balance of genetic and environmental determinants can be represented by a point somewhere within the triangle. The contributions of genetic and environmental factors to human diseases Haemophilia Osteogenesis imperfecta Club foot Pyloric stenosis Dislocation of hip Duchenne muscular dystrophy GENETIC Phenylketonuria Galactosaemia Rare Genetics simple Unifactorial High recurrence rate Peptic ulcer Diabetes Tuberculosis ENVIRONMENTAL Spina bifida Ischaemic heart disease Ankylosing spondylitis Common Genetics complex Multifactorial Low recurrence rate Scurvy Polygenic diseases The most common yet still the least understood of human genetic diseases Result from an interaction of multiple genes, each with a minor effect The susceptibility alleles are common Type I and type II diabetes, autism, osteoarthritis Population Genetics • Identifies how much genetic variation exists in populations • • Investigates factors, such as migration, population size, and natural selection, that change the frequency of a specific gene over time Coupled with DNA technology, investigates evolutionary history and DNA identification techniques Non-Traditional Inheritence Mitochondrial genes Trinucleotide repeats Genetic imprinting Mitochondrial Inheritance • Matrilineal mode of inheritance: only mother • • • • passes mitochondrial DNA to offspring Higher spontaneous mutations than nuclear DNA affects both males and females , but transmitted only through females range of phenotypic severity due to heteroplasmy Example: diabetes mellitus with sensorineuronal deafness Human Genome Project (HGP) Human Genome Project • Initiated by the same laboratories that • • • • brought you thermonuclear devices 1990 taken over by NIH Actually involved sequencing many genomes First draft sequence in 2001, “completed” in 2003 (public effort and Celera Corp.) DNA sequence in any two human beings is 99.9% identical only 0.1% is unique The human Genome project Goals The study of the genome To determine the DNA sequence (exact order of A,T,G,C,) For all the DNA in human To determine which segment of DNA represent individual genes (Protein Coding Unit Model organisms Mapping Human Geneticbased Diseases • Thousands known • Most genes mapped and sequenced OMIM Synopsis of the Human Gene Map (Updated 8 June 2012) Chromosome 1 2 3 4 5 6 7 8 9 10 11 12 Count 1,309 848 710 517 618 793 610 475 503 494 818 702 Chromosome 13 14 15 16 17 18 19 20 21 22 X Y Count 250 426 399 548 770 190 843 337 144 330 724 46 Number of Entries in OMIM (Updated 7 June 2013) : Prefix * Gene description + Gene and phenotype, combined # Phenotype description, molecular basis known % Phenotype description or locus, molecular basis unknown Other, mainly phenotypes with suspected mendelian basis Totals Autosomal X Linked Y Linked Mitochondr ial Totals 13,197 641 48 35 13,921 146 5 0 2 153 3,216 263 4 28 3,511 1,631 136 5 0 1,772 1,779 126 2 0 1,907 19,969 1,171 59 65 21,264 Applications of the Human Genome Project • Genetic testing ( diagnostic, presymptomatic screening, prenatal) • Gene therapy • Pharmacogenomics: Moving Away from “One-Size-Fits-All” Therapeutics Diagnosis and Prevention of Genetic Diseases Diagnosis • Chromosomal Abberations • Single Gene Disorders Preventions • Genetic Counseling • Prenatal Dignosis • Preimplantation Diagnosis New Technologies Technology Advancement Technological Advances ENIAC iPad 1946 2012 Genome Sequencing Technology Applied Biosystems 3730 DNA Analyzers Oxford Nanopore MinION 2002 2012 • Because of major technological advances, the • • cost of sequencing a human genome has fallen rapidly. And within the next 5 years, the cost of sequencing a human genome will be under $1,000 and will take only hours or days. When the cost is low enough, perhaps reading human genomes will be as routine as blood tests and easy enough to be carried out in your doctor’s office.