Boyle's Law
advertisement

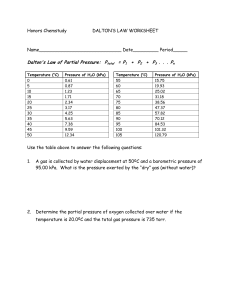
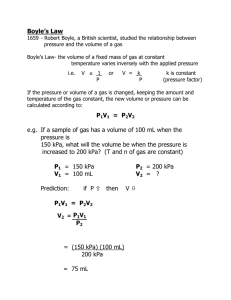
Gas Laws Fundmentals KINETIC MOLECULAR THEORY KMT is a model to explain the behavior of gaseous particles and is based on extensive observations If a gas follows all the ideas of the KMT, it is said to be an ideal gas THE KINETIC THEORY 1. All matter is composed of very small particles 2. These particles are in constant motion 3. Collisions between particles are perfectly elastic PRESSURE Pressure is defined as force per unit area Units: kPa, atm, mmHg, torr VOLUME Volume is defined as the amount of space an object occupies Units: cm3, mL, L DALTON’S LAW Dalton’s Law The physical properties of gases are affected by temperature and pressure John Dalton found that each gas in a mixture exerts pressure independently of the other gases present Dalton’s Law The total pressure of a mixture of gases equals the sum of the partial pressures of the individual gases Ptotal = P1 + P2 + ... When a H2 gas is collected by water displacement, the gas in the collection bottle is actually a mixture of H2 and water vapor Ex. Problem 1: Dalton’s Law What is the total pressure in a container if gas A has a pressure of 13.7 KPa, gas B has a pressure of 5.3 KPa and gas C exerts a pressure of 4.5 KPa? The total pressure in the container is Ptotal GIVEN: Pgas A = 13.7 KPa Pgas B = 5.3 KPa Pgas C = 4.5 KPa Ptotal = PA + PB + PC WORK: Ptotal = PA + PB + PC Ptotal = Ptotal = Ex. Problem 2: Dalton’s Law A mixture of O2, CO2, and N2 has a total pressure of .97 atm. What is the partial pressure of O2, if the partial pressure of CO2 is .70 atm and the partial pressure of N2 is .12 atm. The total pressure is given, you need to find Poxygen. GIVEN: PO = ? 2 PCO = .7 atm 2 PN = .12 atm 2 Ptotal = .97 atm WORK: Ptotal = PO + PCO + PN 2 2 2 BOYLE’S LAW Boyle’s Law Irish chemist (1627-1691) Performed the first quantitative experiments on gases Used j-shaped tube to study the relationship between pressure of trapped gas and its volume Boyle’s Law Boyle’s Law states that at constant temperature the volume of a fixed amount of gas is inversely related to pressure • When the volume INCREASES, pressure DECREASES • When the volume DECREASES, pressure INCREASES Final conditions Boyle’s Law: P1V1 = P2V2 Initial conditions Ex. Problem #1: Boyle’s Law The volume of a gas is 200 mL at a pressure of 100 KPa. What is its volume at a pressure of 200 KPa? P1V1 = P2V2 GIVEN: V1 = P1 = V2 = P2 = WORK: P1V1 = P2V2 Ex. Problem #2: Boyle’s Law If the pressure of the gas in the 4.0 L volume is 200 KPa what will the pressure be at 2.5 L? GIVEN: V1 = P1 = V2 = P2 = WORK: P1V1 = P2V2