Chap5
advertisement
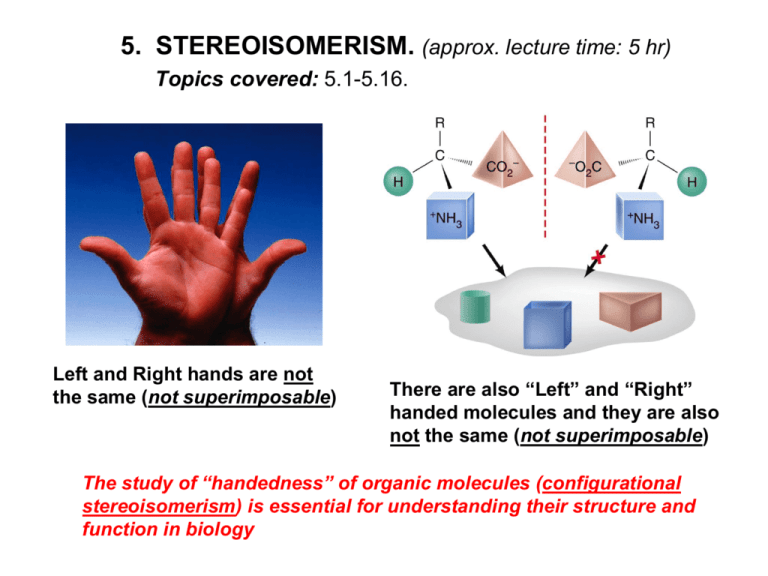
5. STEREOISOMERISM. (approx. lecture time: 5 hr) Topics covered: 5.1-5.16. Left and Right hands are not the same (not superimposable) There are also “Left” and “Right” handed molecules and they are also not the same (not superimposable) The study of “handedness” of organic molecules (configurational stereoisomerism) is essential for understanding their structure and function in biology ISOMERISM (isomers have the same molecular formula but differ in structure) STEREOISOMERISM (stereoisomers have the same connectivity but differ only in the arrangement of atoms in space) CONSTITUTIONAL (STRUCTURAL) ISOMERISM (these isomers have different connectivity of bonds) CIS-TRANS STEREOISOMERISM (for alkenes CONFORMATIONAL STEREOISOMERISM (these are stereoisomers obtained by rotation about sigma bonds; in principle readily interconvertible) and cyclic compounds: stereoisomers that differ in location of atoms on different sides or faces) CONFIGURATIONAL STEREOISOMERISM (stereoisomers obtained by different relative arrangement of 4 bonds on an sp3 hybridized carbon; not interconvertible; maximum number = 2n ; n = number of chirality centers) Configurational Stereoisomerism chirality centre (if W,X,Y,Z are not the same) not superimposable not superimposable (chirality centre = stereogenic centre = chiral centre = chiral carbon) Recognizing Chirality Centers Competing Drugs in the Market VIOXX (Merck) (now withdrawn) VIAGRA (Pfizer) CELEBREX (Pfizer) CIALIS (Eli Lilly) CONFIGURATION STEREOISOMERISM is “independent” of CONFORMATIONAL STEREOISOMERISM Conformational stereoisomers are rapidly interconverting. Configurational stereoisomers DO NOT readily interconvert. (When studying configurational stereoisomerism, we choose a particular “representative” conformational structure, but realizing that many others also exist) Notes on Configurational Stereoisomerism A chiral molecule is one that is not superimposable onto its mirror image structure. The pair of structures are called enantiomers (“left” and “right” hand molecules). Enantiomers have identical physical properties except for the rotation of plane polarized light. Maximum number of configuration stereoisomers possible = 2n (where n is the number of chirality centers in the molecule). There may be less due to the existence of meso compounds. A meso compound is an achiral compound that has 2 or more chirality centers. It is achiral because these compounds have a plane (or center) of symmetry. Diastereomers are stereoisomers that are not enantiomers. This is a widely used term that is not restricted to configurational stereoisomerism. Diastereomers have different chemical and physical properties! A racemic mixture is a 1:1 ratio of the (two) enantiomers. Racemization (to racemize) is the process of converting a solution of one pure enantiomer towards a racemic mixture. Configurational enantiomers and diastereomers are not readily interconvertible. Optical Activity Specific Rotations [a]D (+) rotation “dextrorotatory (d ) (-) rotation “levorotatory (l ) Compound [a]D Compound [a]D Acetic acid 0 Cholesterol -31.5 Chloroform 0 Morphine -132 Sucrose +66.5 Cocaine -16 Camphor +44.3 Methamphetamine +16 (+)-morphine? (+)-cocaine? (dl)-cocaine? or ()-cocaine? Assigning Configuration (R or S) S configuration (Cahn-Ingold-Prelog Rules) 2-Butanol Configuration Stereoisomers assign priorities * Is this R or S? More Examples Additional Rules for Multiple Bonded Substituents Cyclic Compounds enantiomers (not inconvertible) enantiomers (not inconvertible) enantiomers (inconvertible by chair flip! WHY?) Configuration vs Conformational Isomerism R S This is a meso compound when considered FLAT But we know it exists in chair forms. The two chair forms are non-superimposable mirror images and are conformational enantiomers, and rapidly interconvertible, unlike configurational stereoisomers. Conformational Enantiomers (inconvertible by chair flip!) Fischer Projections (How to draw and manipulate molecules with two or more contiguous chirality centres quickly) R R R S S S Interchange any 2 substituents on same carbon results in its INVERSION Rotating molecule by 180 degrees generates the SAME STRUCTURE Rotating molecule by 90 degrees is FORBIDDEN The Aldoteroses (The simple 4-carbon sugars) Thalidomide Babies (1950’s and 1960’s) Thalidomide contains both left- and right-handed isomers in equal amounts. One enantiomer is effective against morning sickness. The other is teratogen (causes birth defects). The two enantiomers of thalidomide The Sex Life of the Olive Fruit Fly (Bacrocera Oleae) Vicks Vapor Inhaler (sold in USA) Active ingredient (R)-(-)-methamphetamine (nasal decongestant). It is the enantiomer of the addictive psychostimulant, (S)-(+)-methamphetamine R S