Ch. 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
advertisement

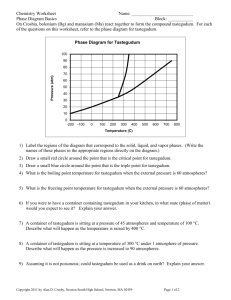
Ch. 12: Liquids, Solids, and Intermolecular Forces Dr. Namphol Sinkaset Chem 200: General Chemistry I I. Chapter Outline I. II. III. IV. V. VI. VII. Introduction Solids, Liquids, and Gases Intermolecular Forces Intermolecular Forces in Action Vaporization and Vapor Pressure Sublimation and Fusion Heating Curves I. Condensed States • Liquids and solids are the condensed states because of the close proximity of atoms/molecules to one another. • This proximity leads to much more frequent interactions than in gases. • Structure determines properties via the nature of the interactions that occur. I. Ethanol vs. Dimethyl Ether • How can the same 9 atoms form two compounds with such different boiling points? • It’s because of the structure of the molecules! I. Electrostatic Forces • Every molecule in a sample of matter experiences two types of electrostatic forces. Intramolecular forces: the forces that exist within the molecule (bonding). These forces determine chemical reactivity. Intermolecular forces: the forces that exist between molecules. These forces determine physical properties. II. Solids, Liquids, and Gases II. Two Types of Solids II. Differences Between the States • A few things stand out when we look at the table. Densities of the condensed phases are much higher. Distances between particles much smaller in the condensed phases. More freedom of movement in liquid than in solid. II. Properties of States of Matter • The state of matter is a “battle” between IM forces and thermal energy (temperature). II. Phase Changes • Phase changes can be induced by adding/removing heat or increasing/decreasing pressure. III. Intermolecular Forces • IM forces originate from interactions between charges, partial charges, and temporary charges on molecules. • IM forces are relatively weak because of smaller charges and the distance between molecules. III. Types of IM Forces • There are different kinds of IM forces, each with a different level of strength. Dispersion force Dipole-dipole force *Hydrogen “bonding” Ion-dipole force III. Dispersion Force • Dispersion force (London force) is present in all molecules and atoms and results from changes in e- locations. III. Instantaneous Dipoles • Charge separation in one creates charge separation in the neighbors. III. Dispersion Force Strength • The ease with which e-’s can move in response to an external charge is known as polarizability. • Large atoms with large electron clouds tend to have stronger dispersion forces. • Larger molecules tend to have stronger dispersion forces. III. Noble Gas Boiling Points III. Dispersion Force and Shape • Molecular size is not the only factor… III. Dispersion Force and Shape • Shape influences how the molecules interact with one another…structure determines properties. III. Dispersion Force in a Family III. Dipole-Dipole Force • Occurs in polar molecules which have permanent dipoles, so attraction is always present. III. Effect of Dipole-Dipole Force • Polar molecules have dispersion forces and dipole-dipole forces. • Effects can be seen in boiling and melting points. III. Increasing Polarity • If we increase the polarity, but keep molar mass approximately the same… III. “Like Dissolves Like” • Polar liquids are miscible (mix without separating) with other polar liquids, but not with nonpolar liquids. • Can be explained with intermolecular forces. III. Hydrogen “Bonding” • This IM force is a misnomer since it’s not an actual bond. • Occurs between molecules in which H is bonded to a highly electronegative element (N, O, F), leading to high partial positive and partial negative charges. • It’s a “super” dipole-dipole force. III. H “Bonding” Ethanol III. Ethanol vs. Dimethyl Ether • We compared these earlier. • Hydrogen “bonding” is so much stronger than dipole-dipole that one is a liquid at room temp. while the other is a gas. III. Effect of H “Bonding” • Hydrogen “bonding” is a very strong intermolecular force. • Without hydrogen “bonding” life as we know it could not exist! III. Ion-Dipole Force • Present in mixtures of ionic compounds & polar compounds. • Example: NaCl(s) dissolved in water. III. Summary of IM Forces III. Sample Problem • Which substance has the highest boiling point and why? a) CH3OH b) CO c) N2 IV. Effect of IM Forces • IM forces lead to a variety of different physical properties. Without IM forces, only gas phase would exist. Surface Tension Viscosity Capillary Action IV. Solid, Liquid, or Gas? • Whether a substance exists as a solid, liquid, or gas depends on the relationship between the intermolecular attractions and the kinetic energy of the molecules. It’s a battle – which dominates? The KE or the IM attractions? Recall that the average KE of a sample is related to its temperature. IV. KE vs. IM Forces • Gas: the kinetic energy of the molecules is much greater than the intermolecular attractions. • Liquid: the kinetic energy of the molecules is moderately greater than the intermolecular attractions. • Solid: the kinetic energy of the molecules is less than the intermolecular attractions. IV. More Dense Objects Can Float IV. Surface Tension • IM force is an attractive force. • More surrounding molecules means more attractive forces and thus lower PE. • Everyone wants to be in the interior. IV. Benefit of Being Spherical • If all molecules want to be interior, then substance will seek lowest surface area. • Surface tension acts as a “skin,” resisting any increase in surface area because that requires energy. IV. Viscosity • Viscosity is the resistance of a liquid to flow. • The unit for viscosity is poise (P) which is defined as 1 g/cm·s. • Stronger IM forces increase viscosity (particles more “sticky”), but temp. can decrease viscosity. • Longer molecules are more viscous because they can become tangled. IV. Sample Viscosities IV. Capillary Action • Capillary action is the ability of a liquid to flow against gravity up a narrow tube. • It is the result of two forces: Cohesive forces (IM forces) Adhesive forces (attractions between molecules and surface of tube). • Need adhesive > cohesive for liquid to flow up the tube. IV. Cohesive vs. Adhesive V. Vaporization and IM Forces • From experience, we know that water evaporates in an open container. • What factors influence rate of vaporization? V. Vaporization Variables • Temperature • Surface area • IM forces V. Energetics of Vaporization • As molecules evaporate, what happens to the temperature of the samples left in the beaker? • Vaporization is an endothermic process – it’s the reason why we sweat when we get too hot. • Condensation is an exothermic process. V. Heat of Vaporization • The energy needed to vaporize 1 mole of a liquid to gas is the heat of vaporization, ΔHvap. • Can be thought of the energy needed to overcome IM forces of the liquid. V. Dynamic Equilibrium • In an open flask, a liquid will eventually evaporate away. • What about a closed flask? V. Dynamic Equilibrium • As evaporation occurs, headspace fills with gas molecules. • Gas molecules condense back to liquid phase. • Eventually, rates become equal. • Pressure of gas at dynamic equilibrium is called the vapor pressure. V. Dynamic Equilibrium • Systems at dynamic equilibrium will seek to return to dynamic equilibrium when disturbed. V. Vapor Pressure and Temp. • Vapor pressure depends on temperature and IM forces. • Why? V. Boiling Point • When T is increased, the vapor pressure increases due to the higher # of molecules that can break away and enter gas phase. • What if all molecules have necessary thermal energy? • At this point, vapor pressure = external pressure, and boiling point is reached. • The temperature at which vapor pressure equals 1 atm is the normal boiling point. V. Boiling Point vs. Altitude V. Boiling Point • At the boiling point, those aren’t air bubbles! V. Pvap – T Relationship V. Clausius-Clapeyron Equation • This equation is in linear form, y = mx + b. • The heat of vaporization can be found using graphical analysis. • Use R = 8.314 J/mole·K. V. Graphical Analysis V. Clausius-Clapeyron Equation, 2-point Form • If you have two sets of pressure, temperature data for a liquid, the more convenient 2-point form of the Clausius-Clapeyron equation can be used. V. Sample Problem • Propane has a normal boiling point of -4.20 °C and a heat of vaporization of 19.04 kJ/mole. What is the vapor pressure of propane at 25.0 °C? V. The Critical Point • In a sealed container, as T of liquid is heated, more and more vapor is formed, and P increases. • At the critical temperature, a supercritical fluid forms; liquid can’t exist above this temperature. VI. Other Phase Changes • Sublimation is the direct conversion of particles from the solid phase to the gas phase. Average KE is low, but always some that have enough KE to break away. • Fusion is the conversion of solid to liquid. • Also have deposition and freezing. VI. Energetics of Fusion • Different compounds have different heats of fusion. • Notice they are much lower than heats of vaporization – why? VII. Energies of Phase Changes • The enthalpies involved in a phase change depends on the amount of substance and the substance itself. • We look at a heating curve for 1.00 moles of H2O at 1.00 atm pressure. • Note that there are sloping regions and flat regions in the curve. (Why?) VII. Heating Curve for H2O VII. Heating Curve, Segment 1 • At this stage, we are heating ice from -25 °C to 0 °C, increasing KE (vibrational motions). • The heat required depends on the specific heat capacity of ice. VII. Heating Curve, Segment 2 • Here, the temperature stays the same, so the average KE stays the same. • Thus, the PE must be increasing. • The heat gained is a factor of the heat of fusion, the heat needed to melt 1 mole of solid. VII. Heating Curve, Segment 3 • During this stage, water is being heated from 0 °C to 100 °C; again, KE is increasing. • The heat gained depends on the specific heat capacity of water. VII. Heating Curve, Segment 4 • Again, the temperature stays the same, so the average KE stays the same. • PE must be increasing. • The heat gained is a factor of the heat of vaporization. VII. Heating Curve, Segment 5 • During this stage, steam is heated from 100 °C to 125 °C; average KE is increasing. • The heat gained depends on the specific heat capacity of steam.