BY 123 SI Session 09/09/15 Chapter 3 Water and Life Water's
advertisement

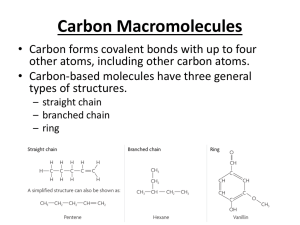
BY 123 SI Session 09/09/15 Chapter 3 Water and Life Water’s having polar covalent bonds leads to what vital force between them? Hydrogen bonding What are the 4 properties of water? Cohesive behavior, moderation of temperature, floats in ice form, solvent of life What is adhesion and surface tension? Be able to envision a solid example of each and to explain each example. Ability of water (due to hydrogen bonds) to attach to atoms OTHER than water such as a straw or the tree trunk walls; Does a coffee pot with boiling water have more heat than a very large swimming pool sitting in 100oF weather? Swimming pool has more heat, volume What’s so special about water’s specific heat? Very high What’s evaporative cooling? What’s heat of vaporization? Examples? The amount of heat needed to evaporate 1g of water = 1 cal. If you lose the quickest molecules on a surface, you reduce temperature (average of energy) What’s the significance of ice floating? How does it float? serves as buffer and habitat for organisms; floats bc water expands as it freezes lending to a lattice structure with many holes leading to its lower density How much larger is the concentration of hydronium ions in a solution with a pH of 2 vs a solution with a pH of 9? a) 7 b) 70 c) 107 d) 710 Chapter 4 Questions L-dopa and R-dopa are enantiomers of the same molecular formula. L-dopa is the correct isomer form that treats Parkinson’s disease. What kind of effect does R-dopa have on Parkinson's disease? A) It alleviates the symptoms. B) It makes the symptoms worse or has no effect on Parkinson’s disease. C) At first it makes the symptoms worse but over the long term it alleviates the symptoms. D) At first it alleviates the symptoms but over the long term it makes the symptoms worse. Structural isomers are molecules that A) are enantiomers. B) are hydrocarbons. C) have a ring structure. D) are mirror images. E) differ in the covalent arrangements of their atoms. Which of these functional groups does not contain oxygen? A) carboxyl B) sulfhydryl C) hydroxyl D) carbonyl E) phosphate The gasoline consumed by an automobile is a fossil fuel consisting mostly of A) aldehydes. B) amino acids. C) alcohols. D) hydrocarbons. E) thiols. Which functional group is most likely to be responsible for an organic molecule behaving as a base? A) hydroxyl B) carbonyl C) carboxyl D) amino E) phosphate Chapter 5 What are the 4 macromolecules? Carbohydrates, lipids, proteins, nucleic acids The 4 macromolecules are all known as polymers as well. T/F Lipids are not polymers because they are not known for being made up of smaller monomers Define polymer. Long molecule consisting of many similar or identical building blocks linked by covalent bonds How are monomers connected to each other? Dehydration or condensation reaction where one monomer provides –OH group and the other provides a hydrogen. How are polymers disassembled to monomers? Hydrolysis reaction where water is added between a bond with h and –oh attaching to the ends of the monomers. How many water molecules are needed to hydrolyze a polymer that is 5 monomers long? 4 Sugars, monosaccharides, and polysaccharides are also called carbohydrates. T/F What is the bond between two conjoined monosaccharides called? Glycosydic linkage If sugars are made up of carbon, hydrogen, and oxygen, how are they all soluble in water? Sugars have many hydroxyl or –OH groups which make them slightly polar and acceptable to the hydrogen bonds in water What are the two types of polysaccharides discussed in class? Storage and structural What are plants’ storage polysaccharides called? What two forms are there? Starch; amylase and amylopectin Animals’ storage polysaccharides? glycogen What are plants’ structural polysaccharides called? Why can’t we digest it? Study figures 5.7 and 5.8. We cannot digest it because we do not have the enzymes to digest beta glucose Why is cellulose so strong, cable-like, and never branched? Because of the structure of beta glucose and the tendency of the hydroxyls to flip each and every glucose molecule, it has the capability of hydrogen bonding to adjacent glucose molecules on the top and bottom of it to make a very tight-knit, cable-like structure. T/F No organism can digest or hydrolyze cellulose. Certain Prokaryotes and protists What are two uses of chitin, a structural polysaccharide? Surgical thread Exoskeleton or found in cell walls of fungi