Section 12.5-12.6
advertisement
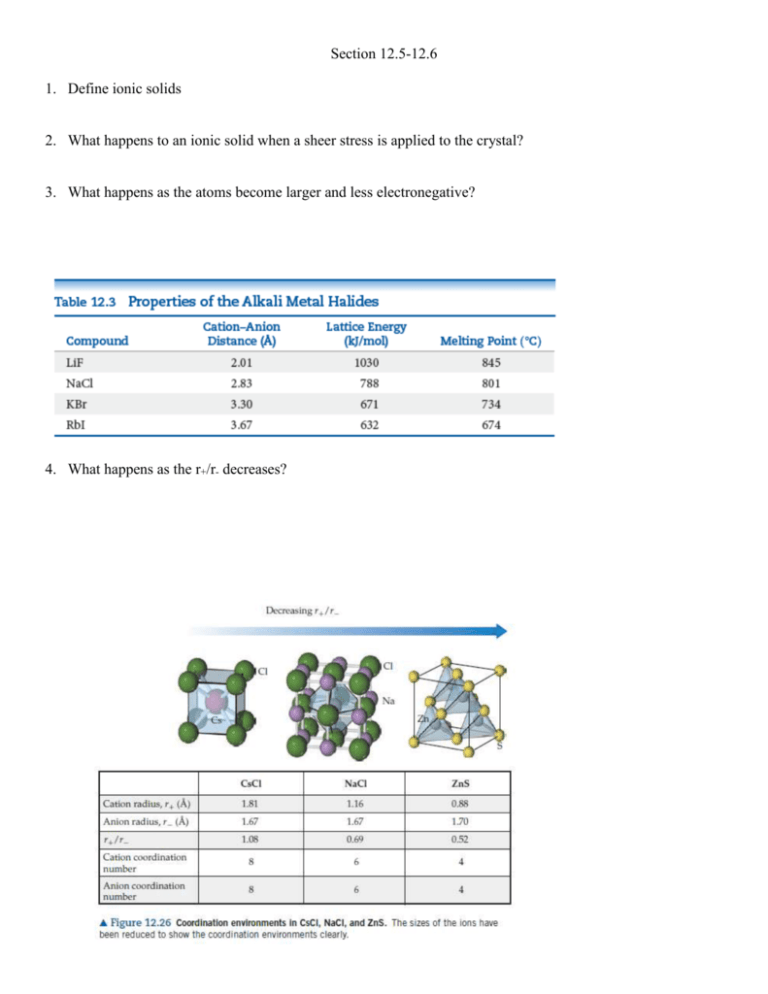
Section 12.5-12.6 1. Define ionic solids 2. What happens to an ionic solid when a sheer stress is applied to the crystal? 3. What happens as the atoms become larger and less electronegative? 4. What happens as the r+/r- decreases? 5. How many cations are per unit cell for each of these structures? How many anions per nit cell? 6. Give equation 12.1 7. Rubidium iodide crystallizes with the same structure as sodium chloride. (a) How many iodide ions are there per unit cell? (b) How many rubidium ions are there per unit cell? (c) Use the ionic radii and molar masses of Rb+ (1.66 Å, 85.47 g/mol) and I- (2.06 Å, 126.90 g/mol) to estimate the density of rubidium iodide in g/cm3. 8. Define molecular solid 9. What are factors that need to be accounted for when determining melting points and boiling points of molecular solids? 10. Tausonite, a mineral composed of Sr, O, and Ti, has the cubic unit cell shown in the drawing. (a) What is the empirical formula of this mineral? (b) How many oxygens are coordinated to titanium? (c) To see the full coordination environment of the other ions, we have to consider neighboring unit cells. How many oxygens are coordinated to strontium? 11. NaF has the same structure as NaCl. (a) Use ionic radii from Chapter 7 to estimate the length of the unit cell edge for NaF. (b) Use the unit cell size calculated in part (a) to estimate the density of NaF. 12. A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.852 Å. (a) Calculate the density of HgS in this form. (b) The mineral tiemmanite (HgSe) also forms a solid phase with the zinc blende structure. The length of the unit cell edge in this mineral is 6.085 Å. What accounts for the larger unit cell length in tiemmanite? (c) Which of the two substances has the higher density? How do you account for the difference in densities? 13. CuI, CsI, and NaI each adopt a different type of structure. The three different structures are those shown in Figure 12.26. (a) Use ionic radii, Cs+ (r = 1.81 Å), Na+ (r = 1.16 Å), Cu+ (r = 0.74 Å), and, I-(r = 2.06 Å), to predict which compound will crystallize with which structure. (b) What is the coordination number of iodide in each of these structures? 14. The coordination number for Mg2+ ion is usually six. Assuming this assumption holds, determine the anion coordination number in the following compounds: (a) MgS, (b) MgF2, (c) MgO. 15. Classify each of the following statements as true or false: (a) Although both molecular solids and covalentnetwork solids have covalent bonds, the melting points of molecular solids are much lower because their covalent bonds are much weaker. (b) Other factors being equal, highly symmetric molecules tend to form solids with higher melting points than asymmetrically shaped molecules.









