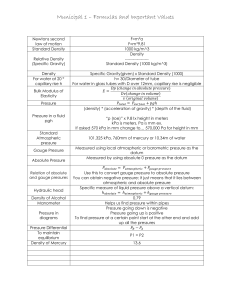
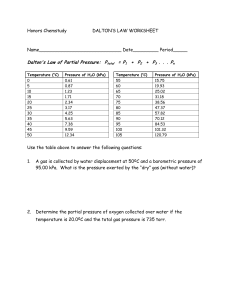
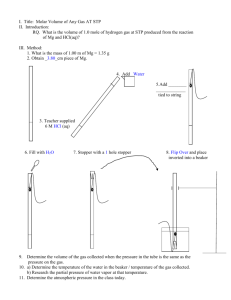
760 mm Hg
advertisement

Needed this week Balloons One and two liter bottles Cotton gloves (to handle and protect from dry ice?) Chapter 13, States of Matter Four states of matter you need to know: Solid Liquid Gas Plasma Determining the states of matter The amount of kinetic energy a substance contains determines its physical state (solid, gas, liquid). The more energy it has, the “looser” it is bound States of Matter Adding Kinetic Energy Notice Did you notice that the Kinetic energy was added in the form of heat? Solids Solids stick together and stay in the same position next to each other (Like a class in which all students are seated) Liquids In a liquid, the particles touch each other but can move freely, like students in a crowded hallway. Gases In a gas, the molecules are not in regular contact with each other and bounce off when they hit one another (picture sixth graders in gym) Gas Pressure Gas pressure is the results of trillions of molecules banging against each other. The pressure is exerted in all directions at once. This is how a balloon or a tire stays inflated. Gas Pressure Atmospheric pressure Atmospheric pressure is the pull of gravity on all the gas above us in the atmosphere. A Barometer measures this pressure At sea level, atmospheric pressure is 760 mm of mercury, on Mt. Everest, it is 253 mm of mercury Barometer Measuring pressure Atmospheres (atm) mm of Mercury (mm Hg) Pascals (Pa) Kilopascals (because pascals are tiny) (kPa) Note = Dumb ole’ TV and radio meteorologists use inches of mercury rather than mm or kPa!!! One inch is 25.4 mm 760 mm = 29.92 inches Converting 1atm equals 760 mm Hg equals 101.3 kPa 1atm = 760 mm Hg = 101.3 kPa Swapping units 450 kPa X 760 mm Hg = 3400 mm Hg 101.3 kPa Note that 760 mm Hg and 101.3 kPa are the same pressure, so you multiply by one to convert Standards STP = Standard Temperature and Pressure 273 K (0° Celsius) standard pressure is 1 atm At STP, one mole of gas occupies 22.4 L Temperature and Kinetic Energy Temperature is the Average Kinetic Energy in a substance Should you need more and better explanations: http://phet.colorado.edu/en/simulation/st ates-of-matter (needs java)(animation) http://www.chemtutor.com/sta.htm (text) http://www.footprintsscience.co.uk/states.htm (animation and text) http://www.youtube.com/watch?v=V9W YweBA6vA (music Video) More links http://www.youtube.com/watch?v=cBB mdqti_Kg (student project video) http://www.youtube.com/watch?v=vDZh Ukp30tE (teacher done music video) Conclusions Answers questions 3-5 on page 389. Click the sounds icon for my answers