Molar Volume of a Gas
advertisement
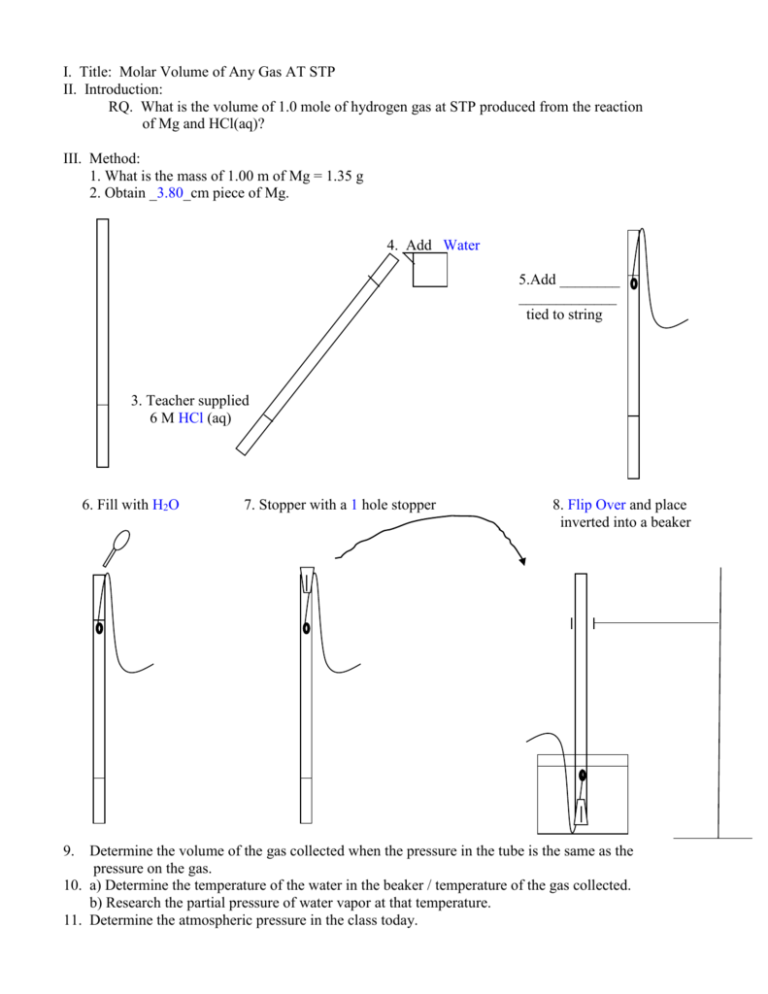
I. Title: Molar Volume of Any Gas AT STP II. Introduction: RQ. What is the volume of 1.0 mole of hydrogen gas at STP produced from the reaction of Mg and HCl(aq)? III. Method: 1. What is the mass of 1.00 m of Mg = 1.35 g 2. Obtain _3.80_cm piece of Mg. 4. Add _Water 5.Add ________ _____________ tied to string 3. Teacher supplied 6 M HCl (aq) 6. Fill with H2O 9. 7. Stopper with a 1 hole stopper 8. Flip Over and place inverted into a beaker Determine the volume of the gas collected when the pressure in the tube is the same as the pressure on the gas. 10. a) Determine the temperature of the water in the beaker / temperature of the gas collected. b) Research the partial pressure of water vapor at that temperature. 11. Determine the atmospheric pressure in the class today. IV. Results The following data can be used to….the volume one mole of any gas would occupy at STP Mass of 1.0 m of magnesium 1.35 g Length of piece of magnesium .36 cm Volume of the gas collected when its pressure equaled the atmospheric pressure 49.9 ml Temperature of gas collected 22.0oC Partial Pressure of water at the temperature the gas was collected 19.8mmHg(101.3 KPa)=2.64Kpa ( 760mmHg) Atmospheric pressure 30.22 in Hg( 25.4 mm ) ( 101.3 kPa ) =102.31kPa ( 1 in ) ( 760 mmHg) V. Conclusion 1. What mass of Mg did you use? .36 cm / 100 cm = x / 1.35 g x = .0486 g 2. How many moles of hydrogen would be produced when it reacts with the excess HCl(aq)? 1 Mg (s) + 2HCl(aq) MgCl2(aq) + H2(g) .0486 g ( 1mole Mg ) ( 1 H2 ) ( ) = .00200 moles H2 ( 24.3g Mg ) ( 1 Mg) ( ) 3. What is the pressure, volume and temperature of the gas collected? V1 = 49.9 ml P1 = PT - Pwater 102.31 KPa – 2.64 KPa =99.67 KPa T1 = ? K 22.0oC + 273 = 295 K 4. What would the volume of this gas be if it were collected at STP? V2 = ? P1V1 = P2V2 T1 T2 P1VIT2 = V2 P2 T1 99.67kPa (49.9mL)273K =45.4mL 101.3KPa 295K P2 = 101.3 KPa T2 = 273 K 5. What volume would one mole of this gas occupy at STP? 45.4 ml H2 = 22,700 ml/ mole Accepted Value = 22,400 ml/ mole or 22.4 L (.00200moles H2) at STP











