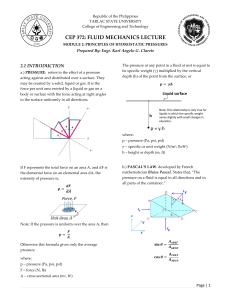
Municipal 1 – Formulas and Important Values Newtons second law of motion Standard Density Relative Density (Specific Gravity) Density For water at 20 ° capillary rise h Bulk Modulus of Elasticity Pressure Pressure in a fluid pgh Standard Atmospheric pressure Gauge Pressure Absolute Pressure Relation of absolute and gauge pressures Hydraulic head Density of Alcohol Manometer Pressure in diagrams Pressure Differential To maintain equilibrium Density of Mercury F=m*a F=m*9.81 1000 kg/m^3 Density -----------------Standard Density (1000 kg/m^3) Specific Gravity(given) x Standard Density (1000) h= 30/Diameter of tube For water in glass tubes with D over 12mm, capillary rise is negligible 𝐷𝑝 (𝑐ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒) 𝐸= 𝐷𝑣(𝑐ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑣𝑜𝑙𝑢𝑚𝑒) 𝑣 (𝑜𝑟𝑖𝑔𝑖𝑛𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒) 𝑃𝑡𝑜𝑡𝑎𝑙 = 𝑃𝑠𝑢𝑟𝑓𝑎𝑐𝑒 + 𝑝𝑔ℎ (density) * (acceleration of gravity) * (depth of the fluid) “ρ (roe)” x 9.81x height in meters kPa is meters, Pa is mm ex. If asked 570 kPa in mm change to… 570,000 Pa for height in mm 101.325 kPa, 760mm of mercury or 10.34m of water Measured using local atmospheric or barometric pressure as the datum Measured by using absolute 0 pressure as the datum 𝑃𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 = 𝑃𝑎𝑡𝑚𝑜𝑠𝑝ℎ𝑒𝑟𝑖𝑐 + 𝑃𝑔𝑎𝑢𝑔𝑒 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 Use this to convert gauge pressure to absolute pressure You can obtain negative pressure; it just means that it lies between atmospheric and absolute pressure Specific measure of liquid pressure above a vertical datum: ℎ𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 = ℎ𝑎𝑡𝑚𝑜𝑠𝑝ℎ𝑒𝑟𝑖𝑐 + ℎ𝑔𝑎𝑢𝑔𝑒 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 0.79 Helps us find pressure within pipes Pressure going down is negative Pressure going up is positive To find pressure at a certain point start at the other end and add up all the pressures 𝑃𝐵 − 𝑃𝐴 P1 = P2 13.6 Municipal 1 – Formulas and Important Values