5H - Chemical Formulas Review
advertisement

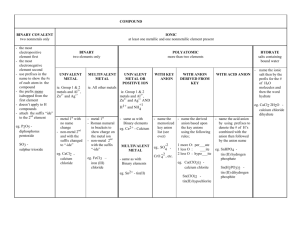
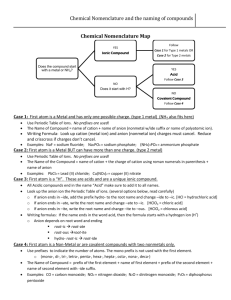
Name: Period: Date: Review: Writing Chemical Formulas Objective 3-3: SWBAT write the formula of an ionic compound. The octet rule tells us that atoms want to have a full octet. In order to do this, they must GAIN or LOSE valence electrons to form IONS. It is difficult to always carry a charge, so ions try to bond with an atom that has an OPPOSITE charge. Sometimes, this means that multiple atoms must bond to even out the charges (see Figure 1). Chemical formulas tell us the NUMBER and TYPE of atoms in a compound. 1 Figure 1 ion bonds with 2 F- ions Mg2+ Steps to Write a Chemical Formula: Step 1: Write the element symbols. The cation always goes first! Step 2: Determine the charge for each ion and write it as a superscript. Step 3: Criss-cross the charges and remove the sign to create the subscripts of the chemical formula. Step 4: Simplify if necessary. Example: Zn2+ and NO3- Zn(NO3)2 How to Find the Charge of a Transition Metal: You need to notice the subscript of the transition metal, subscript of the anion, and CHARGE of the anion. Multiply the subscript of the anion by the charge of the anion. Divide by the subscript of the transition metal. Formula: subscript of anion x charge of anion / subscript of transition metal = charge of transition metal Example: Zn(NO3)2 nitrate is (NO3)- 2 x 1 / 1 = 2 zinc is Zn2+ Practice Directions: Write the chemical formula for the ionic compound that would form. 1. Magnesium and bromine 5. Fluorine and strontium 2. Phosphorous and potassium 6. Boron and nitrogen 3. Calcium and chlorine 7. Caesium and oxygen 4. Aluminum and selenium 8. Copper (2+) and chlorine 9. Nitrogen and lead (4+) 15. Ammonium and selenium 10. Zinc (2+) and sulfur 16. Copper (2+) and hydroxide 11. Titanium (4+) and oxygen 17. Al3+ and F- 12. Phosphorous and cobalt (2+) 18. N3- and Ca2+ 13. Magnesium and nitrate 19. (NH4)+ and O2- 14. Aluminum and sulfate 20. Co4+ and (SO4)2- Directions: Find the charge of the transition metal. 21. Mn2O3 28. TiCl4 22. TiS2 29. CuPO4 23. PbN2 30. Fe2O3 24. CoCO3 31. Ag2O 25. CuSO4 32. AgO 26. NiCl2 33. CuCl2 27. VF5 34. SnBr3