Gurney
advertisement

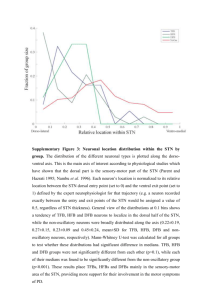
Spiking neuron models of the basal ganglia: dopaminergic modulation of selection and oscillatory properties Kevin Gurney, Mark Humphries, Rob Stewart Adaptive Behaviour Research Group University of Sheffield, UK 1 Rationale: basal ganglia and action selection Aim: to understand underlying function of basal ganglia. While learning is crucial – what is being learned? Hypothesis: Main computational role of basal ganglia is to perform action selection Supported by high (systems) level model 2 Simple leaky integrators to represent population dynamics BUT….. Beyond the systems level Do more realistic models support the selection hypothesis? Constraints provided by: Specific neuronal properties Physiological phenomena displayed by BG in toto…. If the price of a model performing selection is its failure exhibit these phenomena, the selection hypothesis is in question In particular, can models display oscillatory phenomena in BG? If so, then we can use the model to explore possible function of these oscillations 3 Function or artifact?! Systems level – the model architecture Cortex (‘salience’ input) input - + striatum Striatum STN STN output 3 ‘channels’ output nuclei 4 Diffuse projection • Assumes relatively diffuse projection from STN • Emphasises STN’s role as input nucleus cf, Hazrati and Parent, 1992, Mink and Thach 1993, Nambu et al 2000, Sato et al 2000 New functional architecture: selection and control pathways Cortex/thalamus Striatum (D1) STN EP/SNr Gurney et al, 2001 Striatum (D2) GP Interpret GP efferents as control signals for modulating selection pathway Diffuse projection 5 Selection pathway Control pathway Oscillations in basal ganglia: matching mechanisms to phenomena Basal ganglia display a wide range of oscillatory phenomena – from <1Hz to >100Hz These are probably associated with a correspondingly wide range of underlying mechanisms We focus on four BG features. 6 Intrinsic nature of STN-GP coupling Dopaminergic modulation of this coupling Rebound bursting in STN Synaptic patterning Constructing the model 7 Rebound bursting in STN Beurrier et al 1999 Current IT IL IK > IL burst ends IK Time inhibition 8 Importance of synaptic patterning Inhibition at soma or proximal dendrites acts divisively (rather than ‘subtractively’) 70% of GPe input is proximal or somatic (Bevan et al 1997) cortex STN Distal dendrites Proximal dendrites soma GP Captured phenomenlogically: use inhibition in proximal 9 dendrites/soma to explicitly ‘gate’ more distal input Dopaminergic action in striatum Ctx Ctx Glu Glu DA D1 Striatum D2 Striatum decreased PSP Increased PSP W = W0(1 - λ) W = W0(1 + λ) 10 DA λ<1 Dopaminergic action in STN Similar story in GP… GP Ctx D2 DA Glu DA GABA STN STN W = W0(1 – k1 λ) 11 D2 K1, k2 < 1 W = W0(1 – k2 λ) Dopamine: hypotheses Low levels of dopamine serve to couple STN and GP more tightly and to make STN more sensitive to its input Dopamine in striatum will make channel selection easier to achieve 12 Model neurons: summary Leaky Integrate and Fire with 13 AMPA NMDA, GABA, synaptic currents Shunting inhibition at proximal dendrites and soma Spontaneous currents Rebound bursting in STN, Dopamine in striatum, STN and GP. Inter-neuronal delays All of the above parametrised by best estimates from the literature Network Based on systems level model 3 discrete channels 64 neurons per channel, per nucleus Probabilistic connection scheme within channels (only 25% of all possible connections made) 14 Constraining phenomena 1: Low frequency oscillations in STN-GP (Magill et al, Neuroscience,106, 2001) Low frequency oscillations (LFOs) in STN are driven by cortical slow wave under urethane anaesthesia. GP does not oscillate in control (normal DA) conditions. Only shows oscillation under dopamine depletion (6-OHDA lesion) Residual LFOs (with 6-OHDA lesion) in STN and GP under cortical ablation 15 Data – STN control 16 Model - STN control 20Hz Pseudo-eeg STN unit actvity 100 Power Multi-taper spectrum 20 30 mean firing rate (Hz) Bin count 1s 10 17 0 0 0 1 2 3 Frequency (Hz) 4 5 -1.5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 Spike Trig Wav av 20 10 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Data – GP control 18 Model - GP control (1) 20Hz GP unit actvity 50 Power Multi-taper spectrum 50 20 Spike Trig Wav av mean firing rate (Hz) Bin count 1s 25 019 0 0 10 20 30 Frequency (Hz) 40 50 -1.5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 10 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Model GP control (2) 20Hz GP unit actvity 1s 20 Spike Trig Wav av Power Multi-taper spectrum 50 mean firing rate (Hz) Bin count 100 10 20 0 0 0 25 Frequency (Hz) 50 -1.5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Data – STN DA-depleted 21 Model STN DA-depleted 20Hz STN unit actvity Bin count 1s Auto corr. Power 1000 22 0 0 0 1 2 3 Frequency (Hz) 4 5 -1.5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 Spike Trig Wav av 30 mean firing rate (Hz) Multi-taper spectrum 250 20 10 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Data – GP DA-depleted (in phase) 23 Model - GP DA-depleted (in-phase) 20Hz GP unit actvity 1s 2D Graph 7 Power Multi-taper spectrum 24 50 20 mean firing rate (Hz) Bin count 200 100 0 0 0 1 2 3 Frequency (Hz) 4 5 -1.5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 Spike Trig Wav av 10 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Data – GP DA-depleted (anti-phase) 25 Model - GP DA-depleted (anti-phase) 20Hz GP unit actvity Multi-taper spectrum 20 Power 500 50 mean firing rate (Hz) Bin count 1s 26 0 0 -1.5 0 1 2 3 Frequency (Hz) 4 5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 Spike Trig Wav av 10 0 -1.0 -0.5 0.0 Time(s) 0.5 1.0 Data – cortical ablation and DA-depleted Most neurons do not show LFOs but residual LFO activity… 27 100 100 Multi-taper spectrum Power Bin count Model - no cortex (DA-depleted) 50 1s STN 0 -1.5 -1.0 -0.5 0.0 0.5 1.0 0 1.5 0 Time(s) 1 2 3 4 5 Frequency (Hz) GP 1s Bin count 50 Multi-taper spectrum Power Power Multi-taper spectrum 20 25 10 28 0 0 0 0 25 Frequency (Hz) 50 -1.5 0 1 2 3 Frequency (Hz) 4 5 -1.0 -0.5 0.0 Time(s) 0.5 1.0 1.5 LFO counts Neuron is LFO if significant peak in power spectrum below 1.5Hz In DA control conditions, no GP LFOs, STN driven by cortex 100 LFO in GP promoted by DA depletion 50 25 29 GP -ct x Nctx ST GP +c tx tx +c ST N GP -ct x Nctx ST GP +c tx +c tx 0 ST N LFOs (%) 75 Control Data DA dep. data Control Model DA dep. model Residual LFO in STN & GP under cortical ablation Mean firing rates (Hz) Mean firing rates 20 10 30 GP -ct x Nctx ST GP +c tx N+ ctx ST GP -ct x Nctx ST GP +c tx ST N+ ctx 0 Control Data DA dep. data Control Model DA dep. model LFO – mechanistic explanation Low frequency oscillations associated with rebound bursting will be ‘unmasked’ at low levels of dopamine…. GP more likely to generate pre-conditioning hyperpolarisation 31 Constraining phenomena 2: gamma oscillations in STN (Brown et al., Exp Neuro. 177, 2002) There is gamma oscillation (4080Hz) in alert rats This is increased (86% mean) by systemic D2 agonist (quinpirole) Local field potential spectrum (control) 32 Model simulated D2 agonist 60 60 128% power DA=0.2 control → increase 40 Power Power 40 DA=0.8 ‘D2 agonist’ 20 20 Mean power spectra (192 neurons) 0 0 0 20 40 60 80 Frequency 100 0 20 40 Frequency 60 33 No. of sinificant peaks Control DA agonist Peaks in power spectrum 60 40 20 0 0 20 40 60 Frequency (Hz) 80 100 80 100 Gamma oscillations: explanation Gamma oscillations are associated with the natural frequency of oscillation of the GP-STN circuit At control levels of dopamine, the presence of some LFO masks gamma 34 determined by circuit delays Can’t be doing gamma during quiet phase of LFO period. At higher levels of dopamine, gamma is unmasked Selection experiments Cortical input (Mean firing rate) ch2 ch1 1 35 2.5 time Selection and switching ch2 ch3 Mean firing rate SNr ch1 Firing rate Time ch2 ctx 36 ch1 Ctx Ch1: 20 Hz Ctx Ch2: 40 Hz 1 time 2.5 DA depletion prevents selection ch3 ch2 Mean firing rate SNr ch1 Firing rate LFOs? Time ch2 ctx 37 Ctx Ch1: 12 Hz Ctx Ch2: 20 Hz ch1 1 time 2.5 Effects of DA depletion overcome by highly salient action ch2 ch3 Mean firing rate SNr ch1 Firing rate Time ch2 ctx 38 ch1 Ctx Ch1: 20 Hz Ctx Ch2: 40 Hz 1 time 2.5 DA increase results in simultaneous selection ch2 ch3 Mean firing rate SNr ch1 Firing rate Time ch2 ctx 39 ch1 Ctx Ch1: 20 Hz Ctx Ch2: 40 Hz 1 time 2.5 Summary A spiking model of BG constrained by known physiology is able to account for a range oscillatory phenomena Oscillations are modulated under Dopaminergic control of STN and GP The same model displays selection and switching properties, thereby supporting the selection hypothesis for BG function Currently exploring computational role of LFOs 40 Perturb BG to selection in otherwise unresolved selection competition? The adaptive behaviour research group Peter Redgrave Paul Overton Kevin Gurney Tony Prescott Mark Humphries Ben Mitchinson Rob Stewart Ric Wood Jonathan Chambers Tom Stafford 41 Ψ