Review for Moles and Metrics Test
advertisement

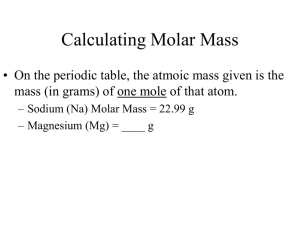
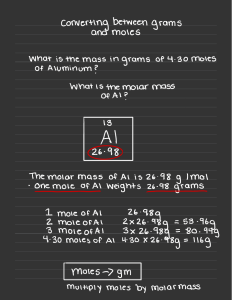
Review for Moles and Metrics Test Question 1 Write out the metric conversion line on your whiteboard _____________________________________ Follow up Which side is has the larger units? Smaller units? Question 2 What are the base units for the following: Time Length Mass Follow up Does the metric conversion line work with any base unit? (Yes or No) Question 3 How many centimeters are in a meter? Follow up How many milliliters are in a liter? Question 4 Convert 5.6 x 103 nm to m. SHOW WORK! Follow up How many nanosecond are there in a second? Question 5 Fill in the correct word or numbers in the blanks below: 1 mole = ____________ molecules/atoms/particles/formula units 1 mole = ____________ Liters 1 mole = ____________ grams/mole Follow up Where can you find the first two answers in the previous question? Question 6 Convert 34.5 L of F2 gas at STP to moles? SHOW WORK! Follow up Does STP affect the problem at all? Follow up #2 Does the molecule (F2) mentioned in the problem affect the answer? Question 7 How many moles are in 8.6 x 1023 atoms of Ca? Follow up Does the atom (Ca) make a difference in how you answer this question? Question 8 Convert 2.4 moles of CO to grams? Follow up Does the molecule (CO) mean anything in this problem? Question 9 What is the formulas for density? Follow up If the molar volume is always 22.4 L, calculate the molar density of CO2? (Think: how do you get molar mass?) Question 10 I want to measure 10 mL of water, what lab equipment should I use to measure this amount out? Follow up Why would I not want to use a beaker? Question 11 What is the difference between accurate and precise? Follow up Describe the measured amounts as accurate, precise, both, or neither. Accepted Amount: 4.5 g Measured Amounts: 6.7 g, 6.6 g, 6.7 g Question 12 How many liters are in 5.4 x 1023 molecules of H2 gas? (TWO STEPS!) Follow up What word was missing in the question that should let you know that it needs to be two steps? Question 13 What is the difference between qualitative and quantitative? Follow up When should you use qualitative? Quantitative?