Stoichiometry Flipbook – Due 3/11/2014 GPS Standard: SC2 All
advertisement

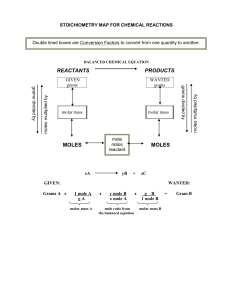
Stoichiometry Flipbook – Due 3/11/2014 GPS Standard: SC2 All students will construct and complete a review booklet in Stoichiometry. Completion of the project will serve as a thorough review of the concepts in these units leading up to the unit test and the semester final/SLO. Front Cover Page: Mole Island and your name in the circle for mole – 10 Points. Page 1: Table of contents – 5 Points- Book is creative and colorful – 5 Points Page 2: Molar mass- 10 Points - Write definition. Write the steps to find the molar mass. Calculate the molar mass of Aluminum sulfate. Page 3: Conversion of mole to gram and vice versa. - 10 Points - Write each step a. Calculate the number of moles in 15 grams of Calcium phosphate. b. Calculate the mass of 15 moles of Barium bicarbonate. Page 4: Conversion of grams to molecules and vice versa. – 10 points - Write each step. a. Calculate the number of molecules present in 25 grams of Sulfuric acid. b. Calculate the mass of 9.4 × 10 26 molecules of Silver nitrate. Page 5: Conversion of grams to volume and volume to grams at STP. -10 points – Write each step a. Calculate the volume of 1.6 grams of Sulfur trioxide at STP. b. Calculate the mass of 1.12 Liters of Chlorine at STP. Page 6: Conversion of molecules to volume and vice versa- 10 Points – Write each step a. Calculate the volume of 9.4 × 10 26 Carbon monoxide at STP. b. Calculate the number of molecules of Carbon dioxide present in 8.96 Liters. Page 7. Percent Composition : 10 points Write the steps to calculate percent composition. Calculate the percent composition of each element in Ammonium sulfate. Page 8: Empirical formula determination – 20 Points a. Write the steps to determine empirical formula from percent composition. b. Determine the empirical and molecular formula of a compound that has the following mass or weight percentages. Cl= 71.65% C=24.27% and H= 4.07% Molar mass of the compound is 98.96 g/mol.