Chemical Quantities Review - Chemistry Honors
advertisement

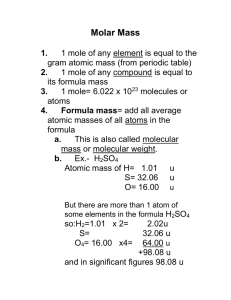
Chemical Quantities Review Chemistry Honors 1. Name the representative particle for the following substances: N ___________________________ CO2 _________________________________________ N+ ___________________________ C6H6 _________________________________________ N2 ___________________________ Na2SO4_________________________ NaHCO3________________________ NaOH _________________________ 2. Calculate the molar mass of the following substances: C6H6 __________________________________ Ca(NO3)2 __________________________________ MgSO47H2O __________________________________ 3. How many moles are in 20.1 g of HC2H3O2? 4. How many molecules are in 100.5 g N2O? 5. How many ions are in 0.67 moles of H2CrO4? 6. Calculate the volume of 67.8 g of Cl2 gas at STP. 7. How many ions are in 0.47 moles of Ca(NO3)2? 8. How many oxygen atoms are in 523 molecules of HC7H5O2? 9. Calculate the volume that 2.46 x 1027 molecules of CO2 gas would occupy at STP. 10. Calculate the mass of 1.98 x 1015 atoms of CH4. 11. Calculate the percentage composition of Ca(OH)2. 12. How many grams of zinc could be extracted from 1.00 kg of Zn(NO 3)2? 13. If the density of a gas is 2.054 g/L, (1) what is the molar mass? (2) identify the gas as either hydrogen, oxygen, nitrogen, sulfur dioxide, fluorine, nitrogen dioxide, or ammonia. 14. Calculate the hydration number for MgSO4·nH2O if 4.89 g of anhydride remain after 10.00 g of hydrate is heated to a constant mass. HELPFUL TIPS FOR THE UPCOMING TEST Know how to calculate empirical and molecular formulas (see today’s notes) Know the four representative particles and be able to determine what the R.P. is for a given substance. Know how to find the molar mass / molecular weight (MW) of a given substance. Know how to determine the number of atoms / ions present in one molecule or formula unit of a given substance. Know how to convert between units of mass, moles, volume, representative particles, and atoms or ions when appropriate. Know how to use gas density to find molar mass. Know the values of standard temperature and pressure. (T = 0C and P = 1atm) Know how to calculate percent composition. Know how to calculate the mass of some part of a compound that could be recovered. o For example, a typical bottle of fingernail polish contains 215.25 mg of tin (IV) oxide. What mass of tin is present in this amount? Begin calculations with the given value. Most of the time you’ll need to convert to the mole before converting to any other unit. Round to the tenth’s place when calculating molar mass. Attach the appropriate units to all values. Give your answer with the same number of significant digits as the given value when converting between units. Use Avogadro’s number (6.02 x 1023 representative particles / mole) to convert to and from representative particles. Use molar mass to convert to and from mass. Use molar volume (22.4 L / mole) to convert to and from volume of a gas at STP. Remember that density = mass (g) / volume (L) Remember: 1 mole = 6.02 x 1023 R.P.’s 1 mole = MW in grams 1 mole = 22.4 L of gas @STP