Phy213_2 - Personal.psu.edu
advertisement

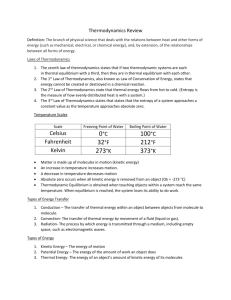
Physics 213: Unit 2 Temperature, Heat, and the Zeroth Law of Thermodynamics Thermodynamics Thermodynamics deals with an internal energy of the systems, the thermal energy, and is governed by the Laws of Thermodynamics Thermal Temperature Zeroth Law of Thermodynamics Temperature in daily life: HOT WARM COOL COLD What is the definition of temperature ? How to measure the temperature? 0th Law 0th Law of Thermodynamics If body A and B are each in thermal equilibrium with a third body T, then they are in thermal equilibrium with each other. Thermal equilibrium: two objects in thermal contact cease to have any exchange of heat. Thermal contact : Heat can be exchanged. Heat: energy exchanged between objects due to their temperature difference. Temperature : two objects in thermal equilibrium with each other are at the same temperature Measuring Temperature Bring a thermometer to a thermal equilibrium with the object Thermometer: physical property changes with temperature — pressure of gas at constant volume — length of solid — Electric resistance — Volume of liquid Temperature Scales DEFINE: Freezing temperature of water: 0˚C Celsius scale Boiling temperature of water: 100˚C Constant-volume gas thermometer: Measure Pressure Temperature Different gases all extrapolate to zero pressure at -273˚C: Kelvin scale Temperature Scales Kelvin scale: T = 0 K for P = 0 point Celsius scale: Tc = T - 273.15˚ Fahrenheit scale: TF = 9/5 Tc + 32˚ Thermodynamic scale: triple point of water T3 = 273.16 K Thermal Expansion When heated, objects expand. Linear expansion: L LT Length changes Original this much length Volume expansion: 3 Temperature changes this much Coefficient of L / L T linear expansion V VT V / V Coefficient of T volume expansion Unusual Behavior of Water The maximum density occurs at 4 ˚C: Above 4 ˚C, expands when heated until 100 ˚C; Below 4 ˚C, expands when cooled until 0 ˚C. Temperature and Heat Internal energy: the energy of a system when it is stationary - nuclear, chemical, strain, etc. Thermal energy: the energy that changes when the temperature changes, associated with motions of atoms, molecules, etc. Heat Q: the transferred energy. Units of Heat Calorie: amount of heat to heat 1 g of water from 14.5 to 15.5 ˚C BTU: amount of heat to heat 1 lb of water from 63 to 64 ˚F SI: Joule (the same as energy) Joule’s experiment: 1 cal = 4.186 J HRW 2E (5th ed.). Suppose the temperature of a gas at the boiling point of water is 373.15 K. What then is the limiting value of the ratio of the pressure of the gas at that boiling point to its pressure at the triple point of water? (Assume the volume of the gas is the same at both temperatures.) P 0 T(K) 0 Constant-volume gas thermometer: P-T curve extrapolates to origin. pb Tb (K ) 373.15 p3 T3 (K ) 273.16 HRW 9E (5th ed.). At what temperature do the following pairs of scales read the same: (a) Fahrenheit and Celsius, (b) Fahrenheit and Kelvin, and (c) Celsius and Kelvin? (a) TF = (9/5)TC + 32˚ For TF = (9/5)TF + 32˚ we get TF = -40 ˚F (b) TF = (9/5)TC + 32˚ = (9/5)(T - 273.15) + 32˚ For TF = (9/5) (TF - 273.15) + 32˚ we get TF = 575 ˚F (c) Since TC = T - 273.15 the Kelvin and Celsius temperatures can never have the same numerical value. HRW 28P (5th ed.). At 20˚C, a rod is exactly 20.05 cm long on a steel ruler. Both the rod and the ruler are placed in an oven at 270˚C, where the rod now measures 20.11 cm on the same ruler. What is the coefficient of thermal expansion for the material of which the rod is made of? The change in length for the rod is 20.11cm-20.05cm plus the expansion of the steel ruler at its 20.11cm mark: ∆Ls = Lss∆T = (20.11 cm)(11 x 10-6 /C˚)(270˚C-20˚C) = 0.055 cm ∆L = (20.11cm-20.05cm) + 0.055 cm = 0.115 cm The coefficient of thermal expansion of the material the rod is made of is: = ∆L/L∆T=23 x 10-6 /C˚ L LT