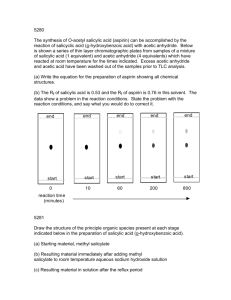
Small Scale Synthesis
advertisement
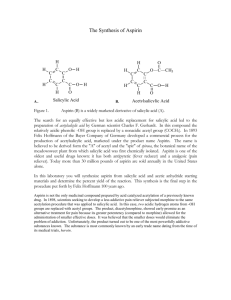
Acetyl Salicylic Acid Synthesis CIBA HS Institute The Main IngredientSalicylic acid H O Lp Lp O H Lp O Lp H An inspection of the structural formula of salicylic acid shows that the molecule consists of the following three units: 1. Phenol Group H H H • Benzene Ring and Hydroxyl group 2. Carboxylic Acid group The Reaction O H Lp H H O O Lp H H + Lp O H Lp H O H O H H H Salicylic Acid H Acetic Anhydride Side Reactions & By-Products O O HO C OH O HO C OH – H2O O HO C HO C – H2O O O C O OH OH C O O polyester polymer represented as HO C OH – H2O – H2O O O HO C O C O O C OH O C O n O •• •• •• •• O O O •• CH3C-O-CCH3 C-OH H O OH •• O •• O C-OH •• •• O O •• •• •• •• O •• HO-CCH3 •• CCH3 •• H •• •• H O •• •• •• •• •• O O •• CH3C-O-CCH3 C-OH H CH3C-O-CCH3 H •• CH3C-O-CCH3 •• C-OH H O O O •• OH •• •• CH3C-O-CCH3 •• •• H O C-OH •• •• •• •• O •• •• O O •• CH3C-O-CCH3 •• CH3C-O-CCH3 H O O •• •• •• •• H H •• •• •• •• + •• •• CH3C-O-CCH3 H H O O •• •• •• •• O •• •• O •• •• •• •• •• Mechanism O O C-OH CCH3 •• O •• Heat (exothermic) O C-O-H O C-CH3 O The Product + Heat ( exothermic) H Lp O Lp O Lp Lp O H H H O H + H H H O H O H H Acetyl Salicylic Acid Acetic Acid H Procedure 1. Place 1.0 g of salicylic acid and 2.5 ml of acetic anhydride in a small reaction vial. Weigh the salicylic acid as it is being added to the vial. Add 5 drops of concentrated Phosphoric Acid to the vial. 2. Seal the vial with a Teflon-coated cap. Make sure the cap is on tight. Shake the reaction mixture. 3. Using tongs, place the sealed vial in the Vial Heating Chamber located on the front bench set at 85 to 95 degrees Celsius. Note the time. Note that the reaction being used is exothermic. 4. As the reaction reaches equilibrium, temp will have an effect on yield for the forward reaction. 4.Place 10 ml of ice and distilled water in a 50 ml beaker. 5.After 10 minutes, use tongs to remove the vial from the Reaction Well and place on the counter for a minute to cool. All the solids should be dissolved before removing the vial from the hot Reaction Well. 6. Remove the cap and pour the contents of the reaction vial into the beaker which contains the ice water . 7. White crystals should form shortly after the reaction mixture is added to the ice water. 8. Rinse the reaction vial with few (1 to 2) mL of distilled water and add it to the beaker. Swirl the beaker with the solution. 9. Add some more ice to the mixture and let stand for about 5-10 minutes. Isolation Of Product • Place the Büchner funnel in a filter flask (fitted with a rubber ring) and stabilize the assembly by clamping the setup to a ring stand. (See figure). • Weigh a piece of filter paper and place it in a Büchner Funnel • Connect the suction hose to a water aspirator and apply suction by turning on the water. Wet the filter paper with some cold distilled water so that it becomes well-seated over the holes in the funnel. • Swirl your sample a bit and pour the mixture onto the moist filter paper in the funnel. Rinse the flask with several small portions of ice-cold distilled water to be sure you get as much product out as you can. • Rinse your final product with about 15 ml of ice-cold distilled water to remove water soluble contaminants. • Let the product air-dry under suction for about 5 minutes. • Carefully lift an edge of the filter paper (use a spatula, tweezers, or stirring rod to get it started if necessary) and remove the paper and contents from the Büchner funnel. Main Reaction O O C-OH OH O O CH3C-O-CCH3 C-O-H + H O O C-CH3 O acetic anhydride salicylic acid aspirin CH3C-OH Data Table Stoichiometry • One mole of Salicylic Acid will Produce One Mole of Acetyl Salicylic • 2-hydroxy Benzoic Acid (Salicylic Acid) 138.12 grams/mol • acetate Benzoic Acid (Aspirin)- 180.17 grams/mol Theoretical Yield O O C-OH C-O-H O OH O + CH3C-OH O acetic anhydride salicylic acid O C-CH3 H CH3C-O-CCH3 O aspirin 2.0 g salicyclic acid 1 mole of aspirin 138.1 g/mole salicyclic acid (limiting reagent) 180.2 g aspirin x x 1 mole salicylic acid = 1 mole aspirin 2.6 grams aspirin Mass of Salicylic Acid Molecular Formula of Salicylic Acid Moles of Salicylic Used Grams C7H6O3 mol Molar Mass of Salicylic Acid g/mol Density of Acetic Anhydride 1.080 g/ml Volume of Acetic Anhydride Used Mass of Acetic Acid Used Molecular Formula of Acetic Anhydride Moles of Acetic Anhydride Used 2.50 ml g C4H6O3 mol Limiting Reagent Mass of Aspirin Synthesized grams Molecular Formula of Aspirin C9H8O4 Molar Mass of Aspirin Moles of Aspirin Synthesized g/mol mol Theoretical Yield of Aspirin Synthesized g Experimental Yield of Aspirin g % Yield