Journal # 39 - Mrs. Dawson's Classroom
advertisement

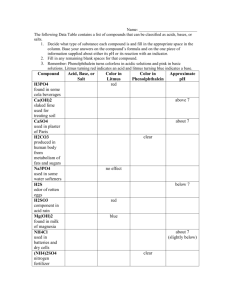
JOURNAL # 62 Write the formula for the following compounds: 1. Sulfur trioxide 2. Iodine trichloride 3. Phosphorus pentabromide 4. Hydrochloric acid TODAY, WE WILL NAME AND ACIDS AND SALTS COVALENT-NETWORK COMPOUNDS Some covalent compounds do not consist of individual molecules Instead, each atom is joined to all its neighbors in a covalently bonded 3-D network. The subscripts indicate the smallest whole number ratio of the atoms in the compound. COVALENT-NETWORK COMPOUNDS Naming such compounds is similar to naming molecular compounds. Examples: SiC SiO2 Si3N4 ACIDS We will further study acids in detail later in the year. Binary acids are acids that consist of 2 elements, usually hydrogen and one of the halogens- fluorine, chlorine, bromine, iodine Oxyacids are acids that contain hydrogen, oxygen, and a third element (usually a nonmetal). Acids usually refer to a solution in water Example: hydrochloric acid refers to a water solution of the molecular compound HCl. ACIDS Many polyatomic ions are produced by the loss of hydrogen ions form oxyacids. Examples: Sulfuric Acid, H2SO4 sulfate Nitric Acid, HNO3 nitrate Phosphoric Acid, H2PO4 phosphate COMMON BINARY ACIDS AND OXYACIDS Name Formula HF Hydrofluoric acid HCl Hydrochloric acid HBr Hydrobromic acid HI Hydriodic acid H3PO4 Phosphoric acid HNO2 Nitrous acid HNO3 Nitric acid H2SO3 Sulfurous acid H2SO4 Sulfuric acid CH3COOH Acetic acid HClO Hypochlorous acid HClO2 Chlorous acid HClO3 Chloric acid HClO4 Perchloric acid H2CO3 Carbonic acid SALTS Salt- an ionic compound composed of a cation and the anion form an acid Example: Table salt, NaCl, contains the anion from hydrochloric acid. Calcium Sulfate, CaSO4 is a salt containing an anion from sulfuic acid. Some salts contain anions in which one or more hydrogen atoms from the acid are retained Such anions are named by adding the word hydrogen or the prefix bi Examples: H2CO3 carbonic acid HCO3- bicarbonate ion or hydrogen carbonate ion LET’S PRACTICE Write the formulas for each of the following compounds: Diphosphorus trioxide Hydrofluoric acid Hydrobromic acid Nitric acid Sulfuric acid Phosphoric acid LET’S PRACTICE Write the formulas for each of the following compounds: H2SO3 HClO3 HCl HClO H2CO3