ionic Bonding
advertisement

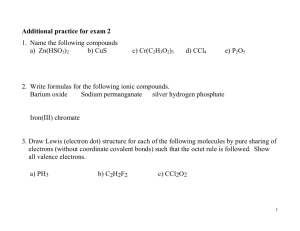
Chemistry 11 Resource: Chang’s Chemistry Chapter 9 Activities Quizzes Exercises Molecular geometry models Lab: Comparing ionic and covalent compounds Lab: Boiling points Objectives Describe the ionic bond as the electrostatic attraction between oppositely charged ions. Describe how ions can be formed as a result of electron transfer. Deduce which ions will be formed by elements in groups 1, 2, 3, 5, 6, and 7. State that transition elements can form more than one ion. Predict whether a compound of two elements would be ionic from the position of the elements in the periodic table or from their electronegativity values. State the formula of common polyatomic ions formed by nonmetals in periods 2 and 3. Describe the lattice structure of ionic compounds. Lewis dot symbols The discovery of the periodic table and electron configuration gave rise to an even greater understanding of chemistry— bonding. Lewis stated that: Atoms combine to achieve greater stability Maximum stability is achieved when the electron configuration resembles that of a noble gas. What properties of noble gases support this? Lewis dot symbols A Lewis dot symbol is a representation of an element. It consists of: The symbol of the element One dot for every valence electron in an atom of the element. What other representations of elements have we used so far? Lewis dot symbols Lewis dot symbol of Lewis dot symbols Draw the Lewis dot symbol for: hydrogen sodium What do you notice? Draw the Lewis dot symbol for: carbon oxygen Ion formation Recall the definition of an ion and the two types of ions. Why do atoms form ions? What roles do you think ionization energy and electronegativity play in the formation of ions? Factor High ionization energy High electronegativity Cation or Anion? Ion formation Explain why atoms form ions using Lewis’s ideas. Factors that affect the formation of ions Factor high ionization energy Cation or Anion? anion low ionization energy high electronegativity low electronegativity cation anion cation Ion formation Predict and explain the ion formation of the following elements: lithium sodium beryllium magnesium aluminum oxygen fluorine Ion formation As a rule: the alkali metals (Group I) and alkaline earth metals (Group II) form cations, and the halogens and oxygen form anions With this in mind, how do you think ionic bonds are formed? The ionic bond An ionic bond is the electrostatic force that holds ions together in an ionic compound. Consider the compound lithium fluoride. Macroscopically, it is a poisonous white powder used in manufacturing. Use your knowledge of electron configuration to describe lithium fluoride at the atomic / molecular level. The ionic bond The electron configurations of lithium and fluoride are: Li 1s22s1 F 1s22s22p5 Lithium surrenders its 2s1 valence electron to fluorine and thus, both achieve the electron configuration of a noble gas. The ionic bond Write the electron configurations of Li and F when they are in an ionic compound. Which noble gases’ configurations do they resemble? The process can also be represented using Lewis dot symbols (p 247) Notice the steps involved in the formation of the ionic bond between Li and F. Ionic compounds What holds ionic compounds together? The ions involved in ionic compounds are electrically charged. Can we then assume that ionic compounds are also charged? Ionic compounds Ionic compounds are held together by the electrostatic force between charged ions, but Ionic compounds themselves are electrically neutral. Lattice structure Consider a very common ionic compound, NaCl (rock salt). What is its gross appearance? The transition metals The transition metals are known to commonly form more than one ion. This comes from the existence of the 3d subshell. Ions of transition metals are commonly of the +2 and +3 charges. Which subshell are the electrons of transition metals taken from? Polyatomic ions It is not uncommon for two nonmetals to join to form a polyatomic ion. Can you predict the charge of the following polyatomic ions? hydroxide (OH) ammonium (NH4) Polyatomic ions One of the elements will be considered as “positive” and the other will be considered “negative”. Some common polyatomic ions Name Molecular formula hydroxide OH nitrate NO3 sulfate SO3 cyanide CN carbonate CO3 ammonium NH4 Charge Objectives Describe the ionic bond as the electrostatic attraction between oppositely charged ions. Describe how ions can be formed as a result of electron transfer. Deduce which ions will be formed by elements in groups 1, 2, 3, 5, 6, and 7. State that transition elements can form more than one ion. Predict whether a compound of two elements would be ionic from the position of the elements in the periodic table or from their electronegativity values. State the formula of common polyatomic ions formed by nonmetals in periods 2 and 3. Describe the lattice structure of ionic compounds. Exercises Chang’s Chemistry p 378