File
advertisement
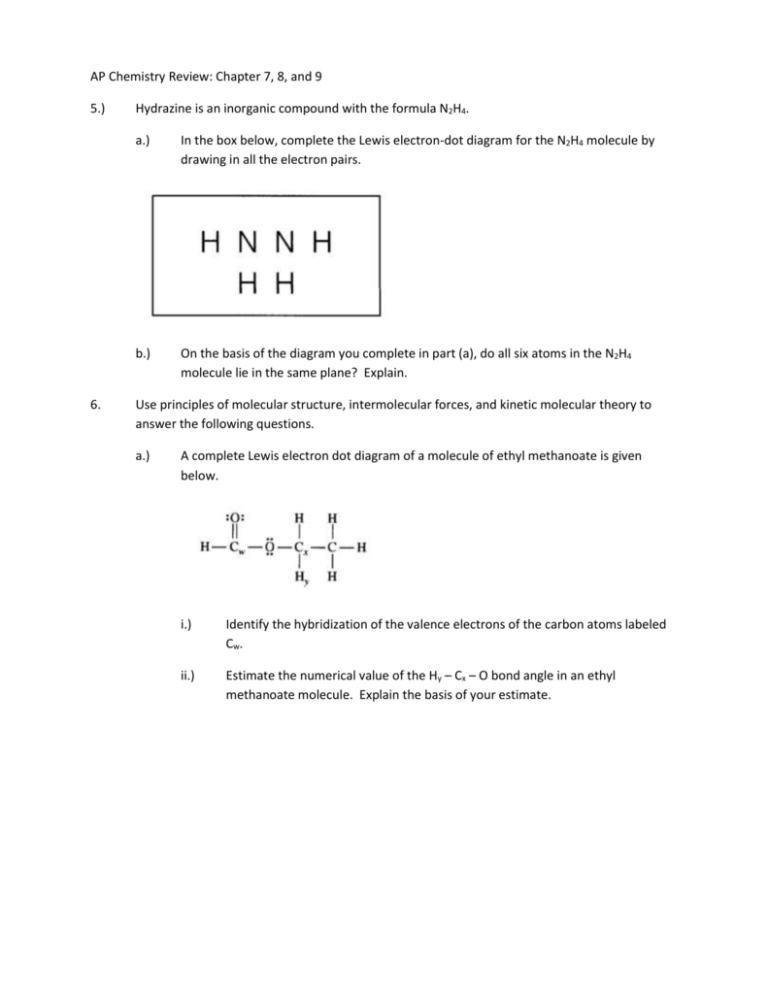
AP Chemistry Review: Chapter 7, 8, and 9 5.) 6. Hydrazine is an inorganic compound with the formula N2H4. a.) In the box below, complete the Lewis electron-dot diagram for the N2H4 molecule by drawing in all the electron pairs. b.) On the basis of the diagram you complete in part (a), do all six atoms in the N2H4 molecule lie in the same plane? Explain. Use principles of molecular structure, intermolecular forces, and kinetic molecular theory to answer the following questions. a.) A complete Lewis electron dot diagram of a molecule of ethyl methanoate is given below. i.) Identify the hybridization of the valence electrons of the carbon atoms labeled Cw. ii.) Estimate the numerical value of the Hy – Cx – O bond angle in an ethyl methanoate molecule. Explain the basis of your estimate. 5. Use the information in the table below to respond to the statements and questions that follow. Your answers should be in terms of principles of molecular structure and intermolecular forces. a. Draw the complete Lewis electron dot diagram for ethyne in the appropriate cell in the table above. b. Which of the four molecules contains the shortest carbon-carbon bond? Explain. c. A Lewis electron dot diagram of a molecule of ethanoic acid is given below. The carbon atoms in the molecule are labeled x and y, respectively. Identify the geometry of the arrangement of atoms bonded to each of the following. i. ii. Carbon x Carbon y 5. Using principles of atomic and molecular structure and the information in the table below, answer the following questions about atomic fluorine, oxygen, and xenon, as well as some of their compounds. Atom First Ionization Energy (kJ mole-1) F 1681.0 O 1313.9 Xe ? a) Write the equation for the ionization of atomic fluorine that requires 1681.0 kJ mol-1. b) Account for the fact that the first ionization energy of atomic fluorine is greater than that of atomic oxygen. (You must discuss both atoms in your response.) c) Predict whether the first ionization energy of atomic xenon is greater than, less than, or equal to the first ionization energy of atomic fluorine. Justify your prediction. d) Xenon can react with oxygen and fluorine to form compounds such as XeO3 and XeF4. In the boxes provided, draw the complete Lewis electron dot diagram for each of the molecules represented below. XeO3 e) XeF4 On the basis of the Lewis electron dot diagrams you drew for part d, predict the following i) the geometric shape of the XeO3 molecule ii) the hybridization of the valence orbitals of xenon in XeF4 GeCl4 SeCl4 ICl4- ICl4+ 6. The species represented above all have the same number of chlorine atoms attached to the central atom. (a) Draw the Lewis structure (electron-dot diagram) of each of the four species. Show all valence electrons in your structures. (b) On the basis of the Lewis structures drawn in part (a), answer the following questions about the particular species indicated. (i) What is the Cl-Ge-Cl bond angle in GeCl4? (ii) What is the hybridization of the I atom in ICl4-? (iii) What is the geometric shape formed by the atoms in ICl4+? 6. Answer the following questions that relate to chemical bonding. a) In the boxes provided, draw the complete Lewis structure (electron-dot diagram) for each of the three molecules represented below. CF4 b) c) PF5 SF4 On the basis of the Lewis structures drawn above, answer the following questions about the particular molecule indicated. i) What is the F-C-F bond angle in CF4? ii) What is the hybridization of the valence orbitals of P in PF5? iii) What is the geometric shape formed by the atoms in SF4? Two Lewis structures can be drawn for the OPF3 molecule, as shown below. i) How many sigma bonds and how many pi bonds are in structure 1? ii) Which one of the two structures best represents a molecule of OPF3? Justify your answer in terms of formal charge. 8. 7. Use principles of atomic structure, bonding, and intermolecular forces to answer the following questions. Your responses must include specific information about all substances referred to in each part. a) Draw a complete Lewis electron dot structure for the CS2 molecule. Include all valence electrons in your structure. b) The carbon-to-sulfur bond length in CS2 is 160 picometers. Is the carbon-to-selenium bond length in CSe2 expected to be greater than, less than, or equal to this value? Justify your answer. c) The bond energy of the carbon-to-sulfur bond in CS2 is 577 kJ mol-1. Is the bond energy of the carbon-to selenium bond in CSe2 expected to be greater than, less than, or equal to this value? Justify your answer. Answer the following questions that relate to the chemistry of nitrogen. a. Two nitrogen atoms combine to form a nitrogen molecule, as represented by the following equation 2 N(g) N2 (g) Using the table of average bond energies below, determine the enthalpy change, ΔH, for the reaction. Bond N–N N=N N≡N Average Bond Energy (kJ mol-1) 160 420 950 6. 2 Al(s) + 3 Zn2+(aq) 2 Al3+(aq) + 3 Zn(s) Respond to the following statements and questions that relate to the species and the reaction represented above. a. Write the complete electron configuration (e.g., 1s22s2…) for Zn2+ b. Which species, Zn or Zn2+, has the greater ionization energy? Justify your answer. 6. Using the information in the table, answer the following questions about organic compounds. Compound Name Compound Formula ΔHvapo (kJ mol-1) Propane Propanone 1-propanol CH3CH2CH3 CH3COCH3 CH3CH2CH2OH 19.0 32.0 47.3 a. For propanone, i. draw the complete structural formula (showing all atoms and bonds) ii. predict the approximate carbon-to-carbon-to-carbon bond angle. c. Draw the complete structural formula for an isomer of the molecule you drew in part a. i. d. Given the structural formula for propyne below, i. indicate the hybridization of the carbon atom indicated by the arrow in the structure above; ii. indicate the total number of sigma (σ) bonds and the total number of pi (π) bonds in the molecule. 6. Answer the following questions related to sulfur and one of its compounds. a. b. Consider the two chemical species S and S2-. i. Write the electron configuration (e.g., 1s22s2 …) of each species. ii. Explain why the radius of the S2- ion is larger than the radius of the S atom. iii. Which of the two species would be attracted into a magnetic field? Explain. The S2- ion is isoelectronic with the Ar atom. From which species, S2- or Ar, is it easier to remove an electron? Explain. 6. First Ionization Energy (kJ mol-1) Element 1 Element 2 Element 3 Element 4 1251 496 738 1000 Second Ionization Energy (kJ mol-1) 2300 4560 1450 2250 Third Ionization Energy (kJ mol-1) 3820 6910 7730 3360 The table above shows the first three ionization energies for atoms of four elements from the third period of the periodic table. The elements are numbered randomly. Use the information in the table to answer the following questions. a. Which element is most metallic in character? Explain your reasoning. b. Identify element 3. Explain your reasoning. c. Write the complete electron configuration for an atom of element 3. d. What is the expected oxidation state for the most common ion of element 2? e. What is the chemical symbol for element 2? f. A neutral atom of which of the four elements has the smallest radius? 6. Answer the following questions, which pertain to binary compounds. a. In the box provided below, draw a complete Lewis electron-dot diagram for the IF3 molecule. b. On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF3 molecule. c. In the SO2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO2 molecule. d. On the basis of your Lewis electron-dot diagram(s) in part (c), identify the hybridization of the sulfur atom in the SO2 molecule.







