Chapter 4 Structure of the Atom
advertisement
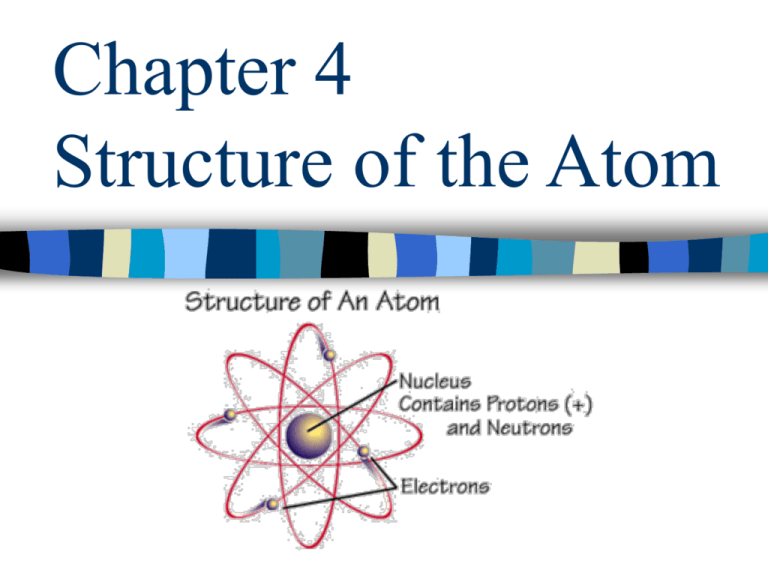
Chapter 4 Structure of the Atom Section 4.1 Democritus (460-370 BC) – Matter is composed of empty space through which atoms move – Atoms are solid, homogeneous, indestructible and indivisible – Different kinds of atoms have different sizes and shapes – The differing properties of matter are due to the size, shape, and movement of atoms – Apparent changes in matter result from changes in the groupings of atoms themselves Aristotle (384-322 BC) – One of the most influential philosophers – Wrote extensively on politics, ethics, nature, physics, and astronomy – Most of his writings have been lost through the ages – Criticized Democritus, saying that he did not believe that the nothingness of empty space could exist John Dalton (1766-1844) – All matter is composed of extremely small particles called atoms – All atoms of a given element are identical. Atoms of a specific element are different from those of any other element – Atoms cannot be created, divided into smaller particles, or destroyed – Different atoms combine in simple whole number ratios to form compounds – In a chemical reaction, atoms are separated, combined, or rearranged The smallest particle of an element that retains the properties of the element is called an atom. Atoms are extremely small (a copper atom has a diameter of 0.000000000128 m) Atoms can be seen using a scanning tunneling microscope. See them on page 96. Section 4.2 J.J. Thomson (1856-1940) discovered the electron through experiments using a cathode ray tube. Robert Millikan (1868-1953) determined that the charge of an electron was negative and was able to calculate the mass of an electron (9.11 x 10-28 g) Earnest Rutherford (1871-1937) is famous for discovering the nucleus of the atom. He used an experiment in which he passed alpha particles through a thin film of gold foil. He expected the alpha particles to pass through, but found instead that they were deflected at large angles. He determined that there was a dense positive core in the atom, which he called a nucleus. Subatomic Particles The nucleus is the tiny positive core of the atom which contains most of the mass of the atom. The proton (p+) is the positively (1+) charged particle found in the nucleus of the atom. It has a relative mass of one. The neutron (no) is the particle with no charge (0) found in the nucleus of the atom. It has a relative mass of one. The electron (e-) is the negatively (1-) charged particle found in the electron cloud outside of the nucleus. It has very little relative mass by comparison. Obj. 5…Subatomic Particles PROPERTIES OF SUBATOMIC PARTICLES PARTICLE SYMBOL Electron e- Proton p+ Neutron n 0 ELECTRICAL CHARGE RELATIVE MASS -1 1/1840 +1 1 0 1 ACTUAL MASS (g) 9.11 x 10-28 1.67 x 10-24 1.67 x 10-24 Since the number of protons is equal to the number of electrons, the atom is electrically neutral Section 4.3 Henry Moseley (1887-1915) discovered that the atoms of each element contain a unique positive charge in their nucleus. The number of protons in an atom is referred to as the element’s atomic number. • Atomic #: # of p+ in an atom identifies element (change atomic # = change of element). # p+ = # e- in neutral atom ** (+) charge = less e- than p+ ** (-) charge = more e- than p+ • Atomic mass: (a.k.a. mass #) mass of the nucleus p+ + n0 units are a.m.u. (atomic mass unit) APEMAN – great shortcut To make it easier, you might want to use APEMAN…. Atomic number = Protons or Electrons Mass number – Atomic number = Neutrons (mass number must be rounded) Since an atom is electrically neutral, the number of protons equals the number of electrons. Atomic # = # of protons = # of electrons Element Protons electrons Pb Atomic Number 82 ? ? ? ? 8 ? ? ? ? 30 Atoms of the same element with the same number of protons, but different numbers of neutrons are called isotopes. All isotopes of any element have the chemical properties of that element. Most elements are a mixture a isotopes. The relative abundance of each isotope is constant. For example, in bananas,93.25% of the K atoms have 20 no, 6.7302% have 22 no, and 0.0117% have 21 no. In any other source of K, the relative percentage of the isotopes will be the same. In order to identify the various isotopes of an element, chemists add a number after the elements name. The number added is called the mass number and it represents the sum of the number of protons and neutrons in the nucleus. Potassium-39 Potassium-40 Potassium-41 P+ No E- 19 20 19 19 21 19 19 22 19 Number of neutrons = mass number – atomic number Because the mass of an atom is so small, chemists have developed a method of measuring the mass of an atom relative to the mass of a specifically chosen atomic standard. The standard is the carbon-12 atom. Scientists assigned the C atom a mass of 12. One atomic mass unit (amu) is defined as 1/12 the mass of a carbon12 atom. 1 amu is approximately equal to the mass of a single p+ or no. Atomic mass of an element is the weighted average mass of the isotopes of that element. Example: The atomic mass of Chlorine (Cl) is 35.453 amu. Chlorine exists naturally as 75% chlorine-35 and 25% chlorine–37. .75770 x 34.969 amu = 26.496 amu .24230 x 36.966 amu = 8.957 amu Weighted average atomic mass of Cl = (26.496 + 8.957) = 35.453 amu • Isotopes are naturally occurring. • The mass # of an element (periodic table) is the weighted avg. of all isotopes that exist in nature. - abundance of isotope is just as important as mass! • Ex... Natural copper (Cu) consists of 2 isotopes ... Copper - 63 (mass = 62 .930 g/mole) 69% Copper - 65 (mass = 64 .930 g/mole) 31% • To calculate avg. mass... mass x abundance for each isotope Step 1 : add the two values from step 1 together Step 2 : 62 .93 x .69 = 43.42 43.42 + 20.13 64 .93 x .31 = 20.13 63.55 g/mole The teacher lied… There are reactions that involve an atom of one element changing into an atom of another element. These reactions, which involve an atom’s nucleus, are called nuclear reactions. Radioactivity is the emission of radiation. Radiation is the radioactive material that is emitted during radioactivity. Radioactive atoms emit radiation because their nucleus is unstable. Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. Types of Radiation Alpha radiation – made up of 2 p+ and 2 no . Is equivalent to He-4 nucleus. During alpha decay, a nucleus loses 4 amu and 2 protons, to become a new element. Beta radiation – consists of fast moving electrons called beta particles. Each beta particle is an electron with a 1- charge. Beta decay changes a n0 into a p+ and an e-. Gamma radiation – Gamma rays are high energy radiation that possess no mass. Because they possess no electrical charge, they are not deflected by electric or magnetic fields. Gamma rays usually accompany alpha and beta radiation and they account for most of the energy lost during the radioactive decay process. Because gamma rays are massless, they cannot result in the formation of a new atom. • Practice: 208 82 230 90 226 88 Pb 0 -1 Th 230 90 Ra 4 2 + 208 83 Th + 0 0 e He + 222 86 Bi beta gamma Rn alpha TYPE ALPHA () BETA () GAMMA () ATOMIC # NEUTRON SYMBOL CHANGE CHANGE 4 2 He +2 by 2 by 2 e by 1 by 1 0 -1 0 0 MASS CHANGE by 4 no change no change no change no change







