PHYSIOLOGY OF RENAL SYSTEM
advertisement

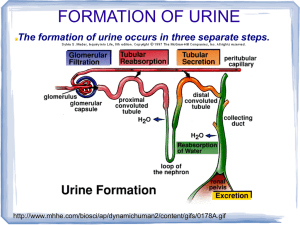
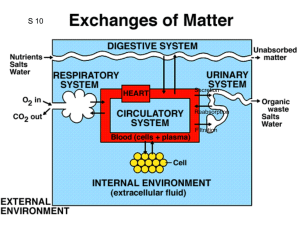
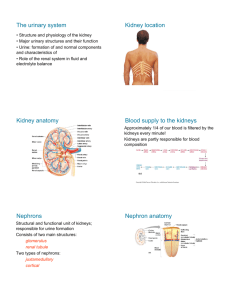
PHYSIOLOGY OF THE RENAL SYSTEM Fredirick L Mashili, MD, PhD. DEPARTMENT OF PHYSIOLOGY, MUHIMBILI UNIVERSITY OF HEALTH AND ALLIED SCIENCES (MUHAS) CONTENTS • • • • • • • • • • • Kidney functions Structure of the kidney Vascularization of the kidney Juxtaglomerular apparatus Glomerular filtration Tubular reabsorption Tubular secretion Ureter physiology Urinary bladder Micturition References KIDNEY FUNCTIONS 1. HOMEOSTASIS • maintain the blood volume and the normal composition of body fluid compartments • excrete waste products ( urea, creatinine, uric acid, NH₃(ammonia), which are toxic for the organism • regulate the blood concentration of ions ( Na+, K +, Cl, Ca++, sulphate, phosphate, bicarbonate) • maintain the pH by secreting the H + • maintain the osmolarity and water volume via the capacity to adjust the water reabsorption • regulate arterial blood pressure • gluconeogenesis KIDNEY FUNCTIONS 2. ENDOCRINE ROLE • synthesis of erythropoetin - sensory cells at the proximal convoluted tubules (PCT), which respond to changes in the partial pressure of oxygen (pO2). • role in metabolism of vitamin D and Calcium - active vitamin D needed to reabsorb Ca²+ in small intestine - to activate vitamin D: an additional hydroxyl group is added => 1.25 dihydroxycolecalciferol - Vitamin D pathway: 1. 7-dehydrocholesterol under the action of UV rays becomes colecalcipherol or vit. D3 ( in skin) 2. Vit. D3 in liver becomes 25 OH D3 and then in kidneys 1,25 (OH)2 D3 or calcitriol → increase Ca absorption in the intestin KIDNEY FUNCTIONS 2. ENDOCRINE ROLE RENIN- ANGIOTENSIN- ALDOSTERON SYSTEM (RAAS) • juxtaglomerular cells (in the wall of the afferent arteriole) synthesize the enzyme RENIN, a glycoprotein with 42000 D , that catalyses the transformation of angiotensinogen (from liver) into angiotensin I. • Angiotensin I is transformed into Angiotensin II ( reaction catalyses by angiotensin converting enzyme – in the lungs) • Angiotensin II causes vasoconstriction (especially in the skin, abdominal organs, kidney (acts on efferent arterioles); less in brain, muscles, heart • Angiotensin II stimulates ALDOSTERONE secretion (in adrenal gland) • Renin is released in case of: renal ischemia (decrease of blood supply to the kidney), decreased blood volume ( due to bleeding, dehydration), hypotension (low blood pressure (BP), cardiac failure RENIN ANGIOTENSIN SYSTEM (after R.Rhoades & G.Tanner, Medical Physiology, 2003) KIDNEY FUNCTIONS 2. ENDOCRINE ROLE • Release prostaglandins E₂(Pg E₂), Pg F2 alpha and Pg I (Prostacyclin)- they act more in a paracrine manner - Pg E₂in hypertensive people : - decrease the blood pressure - increase : - renal blood flow and diuresis (volume of excreted urine/day) - natriuresis (amount of Na excreted via urine/day) - Pg F2 alpha => vasoconstriction STRUCTURE OF THE URINARY SYSTEM The renal apparatus: 1) kidneys (produce urine) 2) urinary excretory pathways • ureters • urinary bladder (it accumulates and stores urine between 2 micturitions) • urethra STRUCTURE OF THE KIDNEY • NEPHRON is the morphological and functional unit of the kidney - there are 1-1.2 millions of nephrons per kidney - it is made up of : 1) renal corpuscle and 2) tubule 1) Renal corpuscle or Malpighian corpuscle/body • Diameter of 200 μm • It is placed in the cortex of the kidney • It consists of : a) Capillary tuft (aprox. 50 capillaries) or glomerulus b) Bowman’s capsule • Role - at its level the process of plasma filtration (glomerular filtration) takes place => primary urine STRUCTURE OF THE KIDNEY 2) Tubule - 45 - 65 mm For reabsorption and secretion processes • a) Proximal convoluted tubule (PCT) o length: 14-12 mm; diameter: 55 μm o one layer of columnar cells on a basal membrane o cells with brush border at the apical pole (towards the lumen) with many microvilli (for reabsorption- increased surface) o cells have invaginations at the basolateral pole (with a striated aspect and many mitochondria) STRUCTURE OF THE KIDNEY b) Loop of Henle • • • • • - like a hairpin it has 2 limbs: ascending and descending, each with a thin and a thick segment 15-20% of glomeruli have long loops of Henle going deep into medulla 26mm Cells: cuboidal in the thick limb and squamous in the thin limb Macula densa: at the final portion of ascending limb the structure is modified (with bigger and fewer cells rich in mitochondria) - Cells with osmo- /chemoreceptors sensitive to Na and Cl concentrations in the urine Juxtaglomerular apparatus: macula densa and juxtaGLOMERULAR cells • When the conc. of Na or Cl in the macula densa decreases => takes place the release of renin from juxtaglomerular cells STRUCTURE OF THE KIDNEY c) Distal convoluted tubule (DCT) o length: 5-8 mm; diameter: 30-40 μm o only a few microvilli, but without a brush border o several distal tubule together become 1 collecting duct (CD), which crosses the cortex and medulla, opens into the renal pelvis and continues into the ureters d) Collecting duct (CD) o across the cortex and the medulla of the kidney o concentrates urine o reabsorption of H2O under the influence of ADH o collection of urine from 3000-5000 nephrons/CD STRUCTURE OF THE KIDNEY(after A.Despopoulos & S.Silbernagl , Color Atlas of Physiology, 2003) VASCULARIZATION OF THE KIDNEY • By the renal artery (directly from the abdominal aorta) -> interlobar arteries (terminal arteries, without anastamosis; in case of an obstruction => necrosis of that territory results) -> arcuate arteries -> interlobular arteries -> afferent arterioles (enter the glomerolus) -> capillary tuft (50) -> efferent arterioles-> peritubular capillaries (loop around the tubules) -> interlobular veins -> arcuate veins -> interlobar veins -> renal vein -> inferior vena cava • 2 networks of capillaries from the efferent arterioles 1) peritubular capillaries (collected by interlobular veins) 2) vasa recta (around the tubules of the juxtamedullary nephrons) • R-I-A-I-A-C-E-P-I-A-I-R • R --- ACEP --- R JUXTAGLOMERULAR APPARATUS Juxtaglomerular apparatus is located in the hillum of every glomerulus • It is made up of juxtaGLOMERULAR cells and the macula densa • Modified muscular layer in the structure of afferent and efferent arterioles: - increased number of smooth muscle cells of afferent arterioles (Af. A) as long as the arteriole comes closer to the glomerulus - cells become thicker (with less actin/myosin filaments, but with many granules containing RENIN) - cells of the juxtaglomerular apparatus act as baroreceptors sensing changes in BP (cells are stimulated by distension of the afferent arteriole; if not distended - release of RENIN) • Low BP (wall of Af. A. is not distended) => Renin secretion => Angiotensin II => increase reabsorption of Na and water => increase BP JUXTAGLOMERULAR APPARATUS (after Vander et al., Human Physiology: The mechanisms of body function, 2001) RENAL CIRCULATION AND ITS REGULATION • Renal circulation has the capacity of autoregulation • It is an intrinsec property of the kidney => it is observed even on isolated, denervated kidney • It is necessary for maintaining a constant GFR and excretion of water and waste products • BP CHANGE OF = 80 –180 mmHg => a constant GFR abd RBF ( renal blood flow) are maintained • It prevents the high variation of water and solutes excretion together with the increase of BP • Autoregulation is explained by two mechanisms: 1) tubuloglomerular feedback mechanism 2) myogenic mechanism AUTOREGULATION OF THE RENAL CIRCULATION 1) Tubuloglomerular feedback mechanism • The increase in BP => increase in GFR => increased NaCl delivery to the macula densa => increased NaCl reabsorption by macula densa cells => constriction of afferent arteriole results • Vasoconstriction can be mediated by : Nitric oxide (NO) , adenosine, ATP • => Renal blood flow (RBF), GFR are lowered to a more normal value. • The tubuloglomerular feedback mechanism = a negative-feedback system that stabilizes RBF and GFR. • Tubuloglomerular feedback mechanism controls the amount of Na presented to distal nephron segments, because these segments have a limited capacity to reabsorb Na. • Renal autoregulation minimizes the impact of changes in arterial blood pressure on Na excretion. • Decreased macula densa sodium chloride causes dilation of afferent arterioles and increased Renin release • Without renal autoregulation, increases in arterial blood pressure => increases in GFR and losses of NaCl and water from the ECF. THE TUBULOGLOMERULAR FEEDBACK MECHANISM G.Tanner, Medical Physiology, 2003) (after R.Rhoades & AUTOREGULATION OF THE RENAL CIRCULATION 2) Myogenic mechanism • The increase in BP => stretches blood vessel wall => opening stretch-activated cation channels in smooth muscle cells => membrane depolarization => opening voltage-dependent Calcium channels => intracellular [Ca] rises => smooth muscle contraction => vessel lumen diameter decreases => vascular resistance increases • Decreased BP induces the opposite changes GLOMERULAR FILTRATION • First step in urine formation (reabsorption and secretion follow) • 25% of the plasmatic renal flow are filtered in the Bowman’s capsule (primary urine) • in resting condition, the tow kidneys receive 1.2-1.3 L/min of blood (=25% of the cardiac output); of this 25% is filtered (only H2O, micromolecules, small proteins, no blood cells or substances bound by plasma proteins) • Primary urine: 180 L/day, with a similar composition as plasma • Final urine: 1.0 – 1.5 L/day, with a composition modified by reabsorption and secretion GLOMERULAR FILTRATION MEMBRANE • Anatomical support for glomerular filtration. Structure (3 layers): • a) Endothelial cells of capillaries - the glomerular capillaries are fenestrated, with holes of 40-100nm in diameter. The endothelial cell surface and the holes are covered with a glycoprotein coat (glycocalyx), about 12 nm thick and with a negative electrical charge • b) Basement membrane has 3 layers : 1. Internal lamina rara 2. Lamina densa (more dense middle layer) 3. External lamina rara It is made up of proteoglycans and collagen fibers, with large spaces through which water and micromolecules can pass. Thickness: 310-340nm • c) The epithelial cells of Bowman’s capsule are named podocytes with many footlike extensions. Podocytes are fixed on the external lamina rara by pedicels. Between the pedicels there is a thin membrane of 4-6 nm in thickness, named slit membrane. - Pores of the slit membrane: 20-30 nm - The surface of the podocytes and slit membrane is covered with glycocalyx. Due to the negative electrical charge, proteins are repelled and their passage into the urine is prevented. This process can be disturbed in many renal diseases (albumin can pass into the urine => albuminuria) GLOMERULUS AND BOWMAN`S CAPSULE ( after A.Despopoulos & S.Silbernagl, Color Atlas of Physiology, 2003) FACTORS INFLUENCING GLOMERULAR FILTRATION (GF) 1) Permeability of the membrane • Some substances have been used for studies of glomerular filtration membrane permeability : - anionic ferritin - 6.1 nm in diameter, negative electrical charge; it cannot pass through the lamina densa of the basement membrane - cationic ferritin passes easier than anionic ferritin, but it is stopped by slit membrane and podocytes - colloidal gold - dextrans (colloid substances used in perfusions to recover BV) • Molecules bigger than albumin (69000 D) are stopped by slit membrane - Plasma albumin: 0.2% passes into urine - Haemoglobin: 5% passes into urine (less negatively charged than albumin) • The smaller and more positively charged the particles are, the easier they can pass through the filtration membrane • Substances with MW < 10000 D can be filtered • Substances bound by plasma proteins ( Ca 2+, free fatty acids) are not filtered • Glomerulonephritis - proteins can pass due to altered permeability (modified glycocalyx amount/structure) => albuminuria FACTORS INFLUENCING GLOMERULAR FILTRATION 2) Surface area of filtration • for the two kidneys is 1.2-1.5 m² • active surface area depends on the number of working nephrons (in humans all nephrons work all the time) • decreased filtration surface/rate: - when mesangial cells among the capillaries of the glomerolus contract (by Angiotensin II and ThromboxanE A₂) - when podocytes are relaxed /flattened they cover more of filtration surface - in many renal diseases reduce the filtration surface (nephrons are destroyed) FACTORS INFLUENCING GLOMERULAR FILTRATION 3) Effective filtration pressure • The volume of filtered plasma depends on the difference between the hydrostatic pressure and the colloid osmotic pressure of the blood and Bowman’s capsule • Pressure of filtration = hydrostatic pressure of the blood (HPB) – hydrostatic pressure of Bowman’s capsule (HPBC) – colloidosmotic pressure of blood (CPB) Filtration pressure = 45mmHg (HPB)- 10mmHg (HPBC)- 25mmHg (CPB) = 10mmHg Hydrostatic pressure decreases with only 1-2 mmHg throught the length of capilllaries, but colloid osmotic pressure increases significantly • Variations - Glomerular filtration => variation if BP changes between 80-180mmHg - Shock/collapse => glomerular filtration decreases (vasoconstriction at the afferent and efferent arterioles). GF stops when BP < 40-50mmHg - Vasoconstriction of the afferent arterioles => decreased glomerular filtration (by CATECHOLAMINES, etc.); at efferent arterioles => increased gomerular filtration (by ANGIOTENSIN II, etc.) - Increased hydrostatic pressure in Bowman’s capsule => impeded filtration (stones, tumors, edema in the renal parenchyma) - Colloidosmotic pressure Decreased => increased filtration (hypoproteinemia) Increased => decreased filtration (hyperproteinemia) REGULATION OF GFR and RBF • GFR is influenced by : 1) sympathetic nervous sytem 2) hormones 3) autacoids 1) Sympathetic nervous system (SNS) Afferent and less efferent arterioles receive sympathetic fibers Strong stimulation of SNS => constriction of afferent A. => decreases RBF => decreases GFR Moderate stim. of SNS => little influence of GFR Its role is more important in : bleeding, shock, ischemia and less in normal conditions REGULATION OF GFR and RBF 2) Hormones • Norepinephrine, Epinephrine constrict renal blood vessels (afferent and efferent A.) and decrease GFR; are released from adrenal medulla. Normally they have little influence on renal blood flow, except some acute conditions (bleeding) • Angiotensin II constricts afferent arteriole; its formation increases in circumstances associated with decreased arterial pressure or volume depletion, which tend to decrease GFR. • The increased level of angiotensin II => constriction of efferent arterioles => increases GFR => maintains normal excretion of metabolic waste products ( urea and creatinine) that depends on GFR for their excretion Angiotensin II, by stimulating the secretion of Aldosteron => increases tubular reabsorption of sodium and water => restores blood volume and blood pressure REGULATION OF GFR and RBF 3) Autacoids • Endotelin - produces vasoconstriction of renal blood vessels - increases in toxemia of pregnancy, acute renal failure, and chronic uremia => decreases GFR • Endothelial-Derived Nitric Oxide (NO) - decreases renal vascular resistance and increases GFR - it is important for maintaining vasodilation of the kidneys - administration of drugs that inhibit this normal formation of NO => increases renal vascular resistance and decreases GFR and urinary sodium excretion => high BP • Prostaglandins (PGE2 and PGI2) and Bradykinin => Increase GFR -Prostaglandins may help prevent excessive reductions in GFR and renal blood flow under stresfull conditions: volume depletion or after surgery - the administration of nonsteroidal anti-inflammatory agents (Aspirin), that inhibit prostaglandin synthesis => reduction in GFR TUBULAR REABSORPTION • During the passage of filtrate through the renal tubule => its composition is changed • Substances move from the tubule to the peritubular capillaries = tubular reabsorption and • from peritubular capillaries to the tubular lumen = tubular secretion • Tubular reabsorption by : - passive transport - active transport TUBULAR REABSORPTION OF GLUCOSE • It takes place at the level of proximal tubules (PCT) • 98% of filtered glucose is reabsorbed at PCT (cca.100g/day) • • • • • • • GFR: 125ML/MIN, normal glycemia: 80-120mg% => Glucose filtered/min: 125mg/min (filtered load) The reabsorbed amount of glucose depends on the amount of filtered glucose and the capacity to transport glucose Glucose transport capacity has a maximum = 375mg/min in males and 300mg/min in females If maximum limit is exceeded => glucosuria (glucose excreted into the urine) Glucose threshold = glucose plasma concentration at which the maximum capacity of reabsorption is exceeded and glucosuria occurs ; It is 180mg% for venous blood and 200mg% for arterial blood Not all nephrons have the same filtration and reabsorption capacity Reabsorption - by symport with Na - at the apical membrane of tubular cells (secondary active transport , mediated by SGLT 1). Glucose diffuses to the interstitial space (by GLT 2) and Na is pumped to the interstitial space (via Na-K-ATPase). Galactose and fructose are competitors for the glucose-symport Renal diabetes - glucose reabsorption is reduced/ impeded ( normoglycemia with glucosuria) Diabetes mellitus -with an overwhelmed glucose reabsorption capacity (hyperglycemia with glucosuria) REABSORPTION OF GLUCOSE AND OTHER ORGANIC SUBSTANCES (after A.Despopoulos & S.Silbernagl, Color Atlas of Physiology, 2003) TUBULAR REABSORPTION OF AMINO ACIDS AND PROTEINS • • • • Amino acids (Aa) are reabsorbed at the level of PCT. Daily 70 g of Aa are filtered . It is similar to glucose reabsorption (Na coupled secondary active transport) Almost complete reabsorption (maximum 1-2% excreted into the urine) There are described several transport systems/ carriers: 1. transport of neutral amino acids (diaminic Aa) 2. transport of proline and hydroxyproline 3. transport of β-amino acids 4. transport of diaminic Aa (arginin, lysine) and dicarboxylic Aa (aspartic acid, glutamic acid) • Defects in reabs. of some Aa => cystinuria (L-cystine, L-arginine and L-lysine are hyperexcreted) => urinary calculus • Proteins- especially albumin, but also lyzozyme, alpha 1-microglobulin, beta 2-microglobulin are filtered - reabsorption - by receptor mediated endocytosis. Proteins are digested by lysosomes inside the cells of the renal proximal tubule, split into aminoacids, which are reabsorpted - this type of reabsorption is nearly saturated at normal filtered loads of proteins => an elevated plasma protein conc. or increased protein sieving coefficient => proteinuria REABSORPTION OF OLIGOPEPTIDES AND PROTEINS (after A.Despopoulos & S.Silbernagl, Color Atlas of Physiology, 2003) TUBULAR REABSORPTION OF UREA AND URIC ACID • • • • • • • • • • • • UREA - daily formed: 25-30g (waste product of protein metabolism) 30-90% reabsorbed (according to diuresis and density of urine) At PCT: 60-65% of water reabsorbed (isoosmotic reabsorption) => urea concentration gradient is obtained daily filtered: 54g of urea => daily reabsorbed: 30g if urinary flow > 2mL/min => 60-70% of urea is reabsorbed Urea reabsorption occurs also at the DCT and CD under ADH action URIC ACID Plasma conc. = 3 –7 mg% It is both reabs. and secreted in PCT waste product of nucleoprotein catabolism daily excreted - 10% of filtrated uric acid = 1g/day alkaline pH => uric acid from urine found as salts (urate - Na urate, K urate) acidic pH => uric acid found as acid (uric acid) => stones formed pH measurement if there is a kidney stone suspicion REABSORPTION OF UREA (after R.Rhoades & G.Tanner, Medical Physiology, 2003) TUBULAR REABSORPTION OF SODIUM • 1kg NaCl is filtered daily • all the segments of the nephron participate in Na reabsorption (except the descending limb of the loop of Henle) • Na reabsorption by diffusion ( along an electrochemical gradient), by symport with glucose and counter transport with H • from cells Na is pumped into the interstitial space (via Na-K-ATPase) • if Na-K-ATPase is blocked by Oubaine => Na reabsorption in the PCT is reduced by 50% • Na ions accumulate in the interstitial space => hyperosmotic environment which attracts H2O to the interstitial space by osmosis (cells at the PCT are permeable to H2O without hormonal intervention -ADH) Cl anions follow Na movement • 65% of filtered Na is reabsorbed at the PCT (along with diffusion of H₂O from the interstitial space to the vessel) • 25% of filtered Na is reabsorbed at the ascending limb of the loop of Henle • 8-10% of filtered Na is reabsorbed at the DCT and CD ION TRANSPORT IN THE THICK ASCENDING LIMB (after R.Rhoades & G.Tanner, Medical Physiology, 2003) CONTROL OF SODIUM REABSORPTION • • • • • Na - the main cation of extracellular fluid It produces 90% of the osmotic pressure of the extracellular fluid compartment Na concentration it is precisely regulated => it gives extracellular fluid volume Na intake is variable - 8-10g/day Renal excretion of Na is regulated by : - ALDOSTERONE - mineralocorticoid hormone produced by thesuprarenal glands - increases Na reabsorption in exchange with K and H, which are secreted - CORTISOL increases Na reabsorption - ATRIAL NATRIURETIC PEPTIDE (ANP) - secreted by atrial myocytes; increases natriuresis (excretion of Na ) and GFR • amount of Na reabsorbed varies proportionately with renal flow • decreased Na or Cl concentrations at DCT stimulate macula densa cells followed by a release of RENIN (by the juxtaglomerular apparatus), => stimulates the formation of ANGIOTENSIN II => stimulates the ALDOSTERONE formation => increases the reabsorption of Na TUBULAR REABSORPTION OF CALCIUM • Ca is not completely filtered because a fraction of plasma Ca is bound to proteins in blood (40%- nonfiltrable) • Daily are filtrated 10000mg • 99.5% of filtered Ca is reabsorbed • 65% reabs. in PCT, 25-30% in LH, 4-9% in DT and CT • Parathyroid gland produces Parathormone (PTH) • PTH increases Ca reabsorption in DCT and CD • Acidosis (proteins bind less Ca) => increased amount of Ca filtered => calciuria TUBULAR REABSORBTION OF CHLORINE • Cl is the main anion of the extracellular fluid • Reabsorption depends to a great extend on Na reabsorption ( Cl follows Na movement) • At the thick ascending limb of the loop of Henle - active Cl transport from the lumen to the cytoplasm along with Na and K via a carrier protein (Na-K-2Cl- transporter) • Inhibition by diuretics e.g. FUROSEMID (inhibits Na reabsorption) TUBULAR REABSORPTION OF BICARBONATE • Not reabsorbed as HCO₃(bicarbonate), because in the presence of H: HCO₃+ H => H2CO₃ and H₂CO₃ => H₂O + CO₂ • CO₂ diffuses from the blood into tubular cells • Acidosis: entire filtered HCO₃is reabsorbed (under acidbase-balance only 99%) • Alkalosis: more HCO₃excreted, less reabsorbed TUBULAR REABSORPTION OF BICARBONATE (after R.Rhoades & G.Tanner, Medical Physiology, 2003) TUBULAR SECRETION OF HYDROGEN • Role - to maintain the normal blood pH • Acids react first with a buffer system => neutralization (mainly by NaHCO₃) • E.g. by RBCs: acid + NaHCO₃ => sodium salt of acid + H₂CO₃ • H₂CO₃ => CO₂ and H₂O • CO₂is removed by respiration and sodium salt removed through the urine • With every mole of filtered urine also 1 mole of NaHCO₃ is lost • Kidneys secrete H ions until the urinary pH of 4.5 is achieved • Secretion of H in PCT, DCT and CD TUBULAR SECRETION OF HYDROGEN • PCT secretion via secondary active transport (antiport/countertransport) with Na • 1 H secreted and 1 Na + 1 HCO₃reabsorbed At apical membrane of tubular cells • DCT • H secreted by active transport (without Na- H-ATPase)- H pumps • H pumps stimulated by ALDOSTERONE (hormone produced by the suprarenal glands; role in reabsorption of Na + H₂O and secretion of H + K) • Acidosis => pumping/secretion of H • Alkalosis => pumping/secretion of K TUBULAR SECRETION OF HYDROGEN • COLLECTING TUBULE • Secretion via 2 types of cells (intercalated and principal cells): 1) Intercalated cells• o similar to the parietal cells of the stomach • o rich in carbonic anhydrase (catalyses CO₂ + H₂O => H₂CO₃) • o luminal membrane with ATPase for H secretion into urine 2) Principal cells production of H in the cells: - by dissociation of H₂O - using CO₂ as a source of H (CO₂ (by cell metabolism) -> blood-> tubular cells where CO₂ + H₂O => H₂CO₃ => H + HCO₃(H secreted in the urine and HCO₃is reabsorbed in the blood)) • H ions in urine react with buffer systems such as HCO₃, phosphates or NH₃to form H₂CO₃, monobasic phosphates or NH₄ • Most H secreted in the PCT reacts with HCO₃ and flows as H₂CO₃ trough urine H₂CO₃ => H₂O and CO₂ (which diffuses back to cells, reacts with H₂O => H₂CO₃ TUBULAR SECRETION OF HYDROGEN • • • • The secretion of H is 3.5 mmole/min (50 mole/day, mostly secreted) Filtration of HCO₃ is 3.49 mmole/min 80-99% of reactions between H and HCO₃occur in the PCT The remaining unbuffered H combines with other buffer systems especially with NaHPO₄ (monobasic sodium phosphate) or Na₂HPO₄(dibasic sodium phosphate) • If urine pH = 5, 90-96% of phosphates excreted via the urine are present as monobasic phosphates • Phosphate ions are the main component of the titratable acidity of the urine • At plasma pH = 7.4, this phosphate system is in the proportion - 1/5 basic (dibasic) and 4/5 acidic (monobasic) components TUBULAR SECRETION OF AMMONIA • 60% from plasmatic glutamine • In tubular cells - glutaminase catalyses glutamine => glutamic acid + NH₃ • Glutamine dehydrogenase catalyses glutamic acid => α-acetoglutamic acid • α-acetoglutamic acid is transformed into NH₄(ammonium) - 40% from other Amino acids (alanine, leucine, lysine, aspartic acid) - NH₃is liposoluble (diffuses from tubular cells) - NH₃ + H => NH₄ (in tubular cells) • NH₄ is hydrosoluble and can’t pass back (remains in urine) • NH₄ + Cl => NH₄Cl • Chronic acidosis: NH₃synthesis increases 10 times within 3-5 days (increases glutaminase activity, even before urine pH changes) RENAL SYNTHESIS AND EXCRETION OF AMMONIA (after R.Rhoades & G.Tanner, Medical Physiology, 2003) TUBULAR SECRETION OF POTASSIUM • • • • • • Daily intake: 50-100 mmole Amount taken should be balanced with the amount excreted 90-95% excreted via urine, 5-10% excreted via GI tract Reabsorption especially at PCT (continued at the ascending limb of the loop of Henle) Secretion at the DCT and CD Secretion takes place in 2 steps 1) Basolateral membrane In the cells of the DCT and CD Via a Na- K-ATPase every free K + 2 Na are reabsorbed 2) Apical membrane • Passive mechanism due to an electrical gradient created by Na reabsorption which favors K secretion (the more Na reabsorbed, the more K secreted) - Secretion stimulated by ALDOSTERONE - Competition between H and K for secretion • Acidosis- increased H secretion • Alkalosis- increased K secretion CHANGES OF URINE OSMOTIC PRESSURE IN THE LOOP OF HENLE • • • • • • • • • • • • Kidneys concentrate urine up to 1400mOsm/L Obligatory diuresis- 0,44 L of H₂O/day to eliminate waste products (600mOsm/day) Independent of fluid intake Desert animals can concentrate urine up to 6000mOsm/L Kidneys can excrete the same amount of solute either via 500ml/day at a concentration of 1400mOsm/L or via 20L/day at concentration of 30mOsm/L At the PCT- H₂O is reabsorbed by osmosis (reabsorption of Na⁺ => hyperosmolarity of interstitial space and causes H₂O to be reabsorbed => isoosmolarity (in the cortex) Change in osmotic pressure due to changes in the osmolarity of urine Descending limb of the loop of Henle: At thedescending limb of the loop of 15% of the total H₂O reabsorbed is reabsorbed here due to a high permeability (at the DCT- 5% reabsorbed , at the CD- 15% reabsorbed ) Urine at the final portion of the descending limb is hypertonic (compared to the interstitial space) Ascending limb of the loop of Henle: Impermeable to H₂O Reabsorption of Na, K and Cl Urine at the final portion of the ascending limb is hypotonic MECHANISMS WHICH MAINTAIN THE OSMOTIC GRADIENT BETWEEN CORTEX AND MEDULLA • Medulla: interstitial space is hyperosmotic (compared to the cortex which is isoosmotic) • Mechanism: counter-current-multiplier-mechanism at the loop of Henle • - Primary urine is isoosmotic (altered by reabsorption and secretion later) • - Descending limb of loop of Henle - increased permeability to H₂O, but decreased permeability to solutes • Hyperosmotic urine (more concentrated) + hypoosmotic interstitial space(IS) • Ascending limb of the loop of Henle - decreased permeability to H₂O, increased permeability to solutes (active reabsorption of Na, Cl, K) • Hypoosmotic urine (less concentrated) + hyperosmotic IS • Na and Cl- reabsorbed in the same proportion as H₂O • Na > H₂O reabsorbed => hyperosmotic IS and hypoosmotic urine MECHANISMS WHICH MAINTAIN THE OSMOTIC GRADIENT BETWEEN CORTEX AND MEDULLA • Hyperosmolarity of the interstitial space maintained => most possible reabsorption of H₂O at the CD • Na from the ascending limb and Na from the hyperosmotic IS go to the descending limb • The longer the loop f Henle, the greater the osmolarity in IS=> increased H₂O reabsorption at the CD • Depends on active transport of Na and Cl out of the ascending limb • Gradient: established by continuous inflow of isotonic solution from the PCT • Gradient against which Na and Cl are pumped out => increased osmolarity in the interstistial space • 50% of the hyperosmolarity is due to urea (suffers change because it diffuses from the medullary part of the CD to the loop of Henle) • Vasa recta: • · Length (20mm) => increased resistance and decreased speed of blood flowing • · Counter-current-exchange- bidirectional-passive (all walls permeable to H₂O+solutes) • · To maintain hyperosmolarity in the interstitial space • · Similar mechanism: H₂O leaves the descending limb of the vasa recta (due to hyperosmolarity in the IS by the loop of Henle) H₂O taken up by the ascending limb (in exchange solvits leave the ascending limb) • Passive process depending on the movement of H₂O and could not maintain the osmotic gradient along the pyramids if the counter-current-multiplier-mechanism at loop of Henle was to cease MECHANISM OF THE URINE CONCENTRATION (after R.Rhoades & G.Tanner, Medical Physiology, 2003) WATER REABSORPTION IN THE COLLECTING TUBULE • H₂O reabsorption under action of ADH (activates aquaporins) • ADH produced by the hypothalamus • Stored in the posterior pituitary gland • Realeased under certain conditions (if increased blood osmolarity, decrease blood volume) • Absence of ADH: 12% of filtered H₂O is excreted (>20L/day) e.g. in diabetes insipidus ACTION OF ADH IN THE COLLECTING DUCT (after R.Rhoades & G.Tanner, Medical Physiology, 2003) WATERY AND OSMOTIC DIURESIS • Watery diuresis Low osmolarity urine After ingestion of hypotonic solutions Diabetes insipidus (with normoglycemia) ADH secretion is reduced Experiment: ADH administration and normal fluid intake to animals => intoxication with H₂O can occur (=>death) • Osmotic diuresis If substances in urine cannot be reabsorbed and keep H₂O from being reabsorbed => increased diuresis and osmolarity of urine Manitol- a polysaccharide filtered but not reabsorbed => increases the volume and the osmolarity of urine Glucose (diabetes mellitus) >180mg% => increased volume and osmolarity of urine URETER PHYSIOLOGY • • • • • • • • • • • • • • • • • Urine formation is a continuous process: 1ml/min urine flows from the kidney to the bladder (urinary flow) Urine flows from the kidney (formation)-> ureters (passage)-> bladder (storage) Ureters 2 tubes with a smooth muscle layer derived from the renal pelvis open into the urinary bladder via the 2 posterior corners of the trigone oblique route/orientation into the bladder wall impedes reflux of urine into the ureters muscular layer in the wall of the ureters has rhythmical contractions/ peristaltic waves · Frequency- 3-6/minute · Speed - 3cm/sec · push urine into urinary bladder Distension of ureters => increased frequency of contractions Ureter continues its activity even if when it is taken off from the organism. It has automatism (have pace maker cells, can work without innervation). The pace maker is placed next to the pelvis Even if they can work without innervation, ureters have a rich sympathetic and parasympathetic innervation Stimulation of sympathetic nerves => inhibition of contractions Stimulation of parasympathetic nerves => stimulation of contractions Stones inside of ureters can produce pain. Pain/Algic impulses induces a uretero-renal reflex, followed by constriction of renal arterioles, decrease or blocking of urine production, by which it is prevent the excessive accumulation of urine in the blocked ureter URINARY BLADDER • It is a muscular, cavitary organ • 2 parts: body and neck (continuous with the urethra) • Trigone- triangle at the posterior wall with the openings of the ureters at the superior corners and more anterior opening of thevurethra • Continuous with the urethra ; in female: 4 - 5cm (increased frequency of urinary tract infections), in men: 20cm • At 2 cm under the neck of the bladder, the urethra passes through the urogenital diaphragm, which forms the external urethral sphincter. It is made up of striated muscle fibers. • Bladder has autonomic and somatic innervation 1) Sympathetic innervation - derive from the lateral horn of the L2 segment of the spinal cord - pass trough paravertebral ganglia chain (mesenteric and celiac ganglia) - form a plexus next to the bladder from where hypogastric nerves (right and left) innervate the bladder (especially the body 2) Parasympathetic innervation derives from S2-S4 segments of spinal cord fibers enter the pelvic nerve to the bladder (body and neck) 3) Somatic innervation for the external urethral sphincter (with striated fibers) derived from the anterior horns of S2-S4 segments of the spinal cord belong to the pudendal nerve afferent signals- nociceptive/pain signals via sympathetic fibers (hypogastric nerve) are transmitted to the spinal cord; touch and stretch signals via parasympathetic fibers (pelvic nerve) INNERVATION OF THE URINARY BLADDER (after R.Rhoades & G.Tanner, Medical Physiology, 2003) FILLING OF THE URINARY BLADDER • within certain limits, the storage of urine in the bladder is not associated with a significant increase of pressure inside the bladder • the graph/plot of intravesical (inside of bladder) pressure related to volume is named cystogram • accumulation of urine up to 400ml with a slight increase in pressure until 10 cm H2O • accumulation of urine in the bladder above 400ml with a high pressure increase => strong urge to urinate • at a volume of 150ml inside the bladder => first desire to urinate • constant pressure between 100-400 ml is due to intrinsic muscle properties of the bladder, based on Laplace`s law : P = 2T/R • law of Laplace: (increased distension => increased pressure) • filling of the bladder increases the radius of the cavity and at the same time the wall tension, without changing the pressure. MICTURITION REFLEX • Micturition- process by which urine is excreted (diuresis- volume of urine excreted daily) • Initiated by stimulation of mechanoreceptors (distension of bladder wall) • Stimulus (increased pressure) -> stimulates mechanoreceptors-> sensitive fibers (pelvic nerve, parasympathetic) -> center at the spinal cord, segments S2-S4 -> efferent fibers (pelvic nerve, parasympathetic) • Nervous impulses are also transmitted through the ascending pathways to the brain stem, hypothalamus and cortex. • If the neurons of the medullary center are not inhibited by superior centers they cause contraction of the detrusor muscle => urine is pushed into the posterior urethra MICTURITION REFLEX • This stimulates receptors in the posterior urethra => transmission of inhibitory signals to the anterior horns of the spinal cord (via the pelvic nerve) => inhibition of the pudendal nerve => relaxation of the external urethral sphincter => micturition • Also supported by contractions of abdominal muscles • After initiation of micturition the reflex is self-mantaining • Initial contraction of the bladder stimulates mechanoreceptors => generation of intense impulses => stronger contractions • Remaining urine can be a risk factor for infections (may be due to disfunctions, prostate hypertrophy) • Also increased pressure can cause retrograde movement of urine => impedes filtration => (hydro-) nephrosis (edema of kidney) • Via medullary centers for the micturition reflex (under control of the superior centers) one can stop micturition voluntarily • Up to the 18th month: micturition is a medullary reflex only • After 2 years: cortical control of micturition (development of the pyramidal tract, which is completely myelinated at 2 years) • Adults have the capacity to maintain the extearnal sphincter contracted until the environmental conditions allow the urination. When the intravesical urinary volume is more than 700 mL, the micturition becomes painful and imperious. REFERENCES • • • • • • Despopoulos A, Silbernagl S, Color Atlas of Physiology, 5th edition, Thieme, 2003 Dorofteiu M, Mecanismele homeostazei sanguine, Editura Dacia, ClujNapoca, 1989 Ganong W.F, Review of Medical Physiology, 22nd edition, The McGrawHill Companies, 2005 Guyton A.C, Hall E.J, Textbook of Medical Physiology, 11th edition, Elsevier Saunders, Philadelphia, 2006 Rhoades R, Tanner G, Medical Physiology, 2nd edition, Lippincott Williams & Wilkins, 2003 Vander et al, Human Physiology: The Mechanism of Body function, 8th edition, The McGraw Hill Companies, 2001