prefixes
advertisement

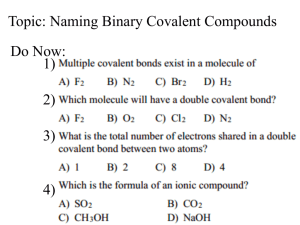
Binary Covalent Compounds • Composed of two non-metals –can be same or different Two naming systems • Stock system: – learned for ionic compounds – “official” • Traditional: – what hear on news – what see on ingredient lists Both Traditional & Stock • Least electronegative of two elements goes first • See table S Stock System 1st element must have Roman Numeral so… figure out oxidation number Stock System & Oxidation Number • molecular substances DON’T contain ions • concept of oxidation # for e- bookkeeping –pretend all shared e- go to atom with higher electronegativity value • atom with higher electronegativity value: (-) oxidation # • atom with lower electronegativity value: (+) oxidation # 7 Rules for Oxidation Numbers 1. # for free, uncombined element is 0 Na He O2 N2 S8 Cl2 P 2. # for monatomic ion = charge on ion Ca+2 = +2 Cl-1 = -1 Al+3 = +3 3. Fluorine is always (-)1 CF4 4. Hydrogen is nearly always (+)1, except when bonded to metal, then (-)1 LiH CaH2 NaH 5. Oxygen is nearly always (-)2 EXCEPT when: OF 2 -Bonded to fluorine: O is (+)2 -In peroxide ion: O is (-)1 O22- 6. sum oxidation numbers in neutral compound is 0 H2O CO2 NO SO3 7. sum of oxidation numbers in polyatomic ion = charge of ion Sum in SO42- = -2 Sum in NO31- = -1 Naming Binary Covalent Compounds Stock System CO2 CO2: C goes first: less electronegative than O so C is +4 Each O is -2 CO2 Total = +4 carbon (IV) oxide Total = -4 Try SO3 so S is +6 Each O is -2 SO3 Total “pos” = +6 sulfur (VI) oxide Total “neg” = -6 Try N2O3 Each O is -2 Each N is +3 N2 O 3 Total “pos” = +6 nitrogen (III) oxide Total “neg” = -6 Try P2O5 Each O is -2 Each P is +5 P2O5 Total “pos” = +10 Total “neg” = -10 phosphorus (V) oxide Naming Binary Covalent Compounds Traditional Naming System Traditional Naming 1. less electronegative element named 1st 2. stem 2nd element plus “-ide” ending 3. prefixes tell how many of each element Exception: NEVER start a name with “mono-” Prefixes: Traditional System # atoms prefix # atoms prefix 1 mono 6 hexa 2 di 7 hepta 3 tri 8 octa 4 tetra 9 nona 5 penta 10 deca Traditional Naming H2O dihydrogen monoxide NH3 nitrogen trihydride N2H4 dinitrogen tetrahydride NO nitrogen monoxide NO2 nitrogen dioxide N2O dinitrogen monoxide