Tubular Proteinuria
advertisement

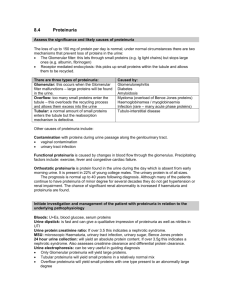
DR.Sh.Sajjadieh nephrologist Abnormal quantity of protein excretion in the urine is called proteinuria. 95% of normal adults excrete less than 150 mg/d. Children and adolescent can excrete up to 250 mg/d. Higher rates of protein excretion that persist beyond a single measurement should be evaluated. 3 Proteinuria is defined as urinary protein excretion exceeding 2 SD from the mean. Normal values depend upon the age Premature neonates full term neonates Children <10 yr Children 10-18 yr Adults 140 mg/m2/d 70 mg/m2/d 150 mg/d 300 mg/d 150 mg/d 30 to 150 mg/day Usually only small proteins (<20,000 daltons) pass across capillary wall and most are reabsorbed in prox. tubules e.g., a2-microglobulin, apoproteins, enzymes, peptide hormones Tamm-Horsfall protein (uromodulin) High m. wt. 23 x 106 daltons glycoprotein Thick ascending limb and distal convoluted tubule IgA and urokinase in small amounts Disruption of glomerular capillary barriers (high m. wt. proteinuria) Tubular damage, inability of prox. tubule to reabsorb normally filtered proteins (low m. wt. proteinuria) Increased production of normal and abnormal plasma proteins which are filtered and inadequately reabsorbed Increased tubular production of protein PH indicators change color with protein Sensitive for albumin but not immunoglobulins Strongly pigmented urine interferes with color reaction (hyperbilirubinemia, pyridium) False positives in very alkaline urine Sensitivity, 32-46% using 25 mg/dL proteinuria Cold precipitation of proteins with a strong acid urine will become turbid, measured by photometry Poor precision - coefficient of variation is 20% Gamma globulins and albumin detected, more sensitive to the latter Substituting trichloracetic acid results in more sensitivity for gamma globulins Turbidity of urine from another cause Radiographic contrast dye (high SG on urinometer but normal dipstick) Penicillin, cephalosporin, sulfonamides, tolbutamide, tolectin False negative in alkaline urine If SSA is positive while dipstick reagent is negative - non albumin proteinuria is suspected (e.g., gamma globulin paraproteins) -Pancreatic transplant patients, nonalbumin pancreatic enzymes in urine Radioimmunoassay – double antibody technique Immunoturbidometric technique - albumin + antibody create a turbid mixture measured by spectrophotometry Albumin - antibody complexes measured by laser nephelometer ELISA Urinanalysis 70-80% of excreted protein is in upright position. The urine dipstick for protein primarily detects albumin. 15 Urinanalysis In normal urine, 60% of the proteins originate from the plasma and other 40% from the kidney and urogenital tract. Normal protein composition is approximately 40%albumin, 40% TommHorsfal protein, 15% immunoglobulins, 5% other plasma proteins. 16 Urinanalysis Dipstick is simplest and least expensive method. It detects protein concentration > 20 mg% and depends on urine concentration and is insensitive to globulin. False positive in highly alkaline urine, presence of bacteria, blood, ammonium compounds, or chlorhexidine. 17 Urinanalysis Sulfosalicylic acid is more sensitive (down to 5 mg%) and react equally with albumin and globulin. 18 Urinanalysis False positive results can occur if the urine contains tolbutamide, radiocontrast agents, or high level of cephalosporin, penicillin, or sulfonamides. 19 Urinanalysis Quantitatively method is urine electrophoresis. At times, it is necessary to quantify protein excretion; determining the 24-hour protein collection, or protein to creatinine ratio. 20 Urinanalysis Microalbuminuria refers to elevated urine albumin excretion that is below the level of detection (30-300 mg/d or 20-200 mg/min) by routine urine protein dipstick test. 21 There are three basic types of proteinuria : glomerular, tubular, and overflow. Only glomerular proteinuria (ie, albuminuria) is identified on a urine dipstick. Glomerular proteinuria : the proteinuria in glomerular disease is due to increased filtration of macromolecules (such as albumin) across the glomerular capillary wall. 23 Low molecular weight proteins ( such as microglobulin, immunoglobulin light chains, retinol-binding protein, and amino acids) have a molecular weight that is generally under 25,000 in comparison to the 69,000 molecular weight of albumin. 24 Tubular Proteinuria These smaller proteins can be filtered across the glomerular and are then almost completely reabsorbed in the proximal tubule. 25 Tubular Proteinuria Interference with proximal tubular reabsorption, due to a variety of tubulointerstitial diseases or even some primary glomerular diseases, can lead to increased excretion of these smaller proteins. 26 Tubular proteinuria is not diagnosed clinically since the dipstick for protein does not detect low molecular weight proteins and the quantity excreted is relatively small. Characteristic Tubular Glomerular Sp. Gravity Isosthenuric High (conc.) Protein 1+ on dipstick 3+ Hematuria Usually absent Often present Casts Tubular cells/ none Variable (RBC, granular UPEP Broad band – multiple globulin Albumin peak Increased excretion of low molecular weight proteins can occur with marked overproduction of a particular protein (almost always immunoglobulin light chains in multiple myeloma) In this setting, the filtered load is increased to a level that exceeds the normal proximal reabsorptive capacity. 29 Abnormal protein excretion due to tissue or tumor necrosis. 30 Proteinuria without hematuria or an elevated serum creatinine concentration asymptomatic and the presence of proteinuria is discovered incidentally. This is different from that in patients with more prominent renal disease who have one or more of the following: heavy proteinuria (>3 g/day), lipiduria, edema, and/or an active urine sediment containing red cells (which are often dysmorphic) and red cell casts. Annual screening for proteinuria is not costeffective in the general population of healthy individuals under age 60. Routine urinalysis is recommended for high risk patients :diabetes or hypertension Early detection of proteinuria in high risk patients is important because the administration of an ACE inhibitor or ARB → slow the progression of proteinuric chronic kidney disease. Almost all cases of persistent proteinuria are due to glomerular proteinuria. Increased filtration of macromolecules (such as albumin) across the glomerular capillary wall : diabetic nephropathy and other glomerular diseases orthostatic or exercise-induced proteinuria Most patients with benign causes of isolated proteinuria excrete less than 1 to 2 g/day Low molecular weight proteins :ß2-microglobulin, immunoglobulin light chains,, and amino acids — have a molecular weight that is generally under 25,000 can be filtered across the glomerulus and are then almost completely reabsorbed in the proximal tubule. The increased excretion of immunoglobulin light chains (or Bence Jones proteins) in tubular proteinuria is mild, polyclonal (both kappa and lambda), and not injurious to the kidney. This is in contrast to the monoclonal and potentially nephrotoxic nature of the light chains in the overflow proteinuria seen in multiple myeloma. Increased excretion of low molecular weight proteins can occur with marked overproduction of a particular protein, leading to increased glomerular filtration and excretion. This is almost always due to immunoglobulin light chains in multiple myeloma, but may also be due to lysozyme (AML), myoglobin (in rhabdomyolysis), or hemoglobin (in intravascular hemolysis) . Some patients have mixed forms of proteinuria: Patients with myeloma kidney also may develop a component of tubular proteinuria, since the excreted light chains may be toxic to the tubules, leading to diminished reabsorption. Glomerular diseases such as focal segmental glomerulosclerosis can be associated with proximal tubular injury, leading to tubular proteinuria. Standard urine dipstick Detection of albumin via a colorimetric reaction between albumin and tetrabromophenol blue producing different shades of green according to the concentration of albumin in the sample. The dipstick is insensitive to the presence of nonalbumin proteins. positive dipstick usually reflects glomerular proteinuria. Pure tubular or overflow proteinuria will not be diagnosed unless a 24-hour urine is collected for some other reason, or the urine is tested with sulfosalicylic acid which detects all proteins. Both the dipstick and sulfosalicylic acid test will detect urinary lysozyme (AML). Thus, lysozyme excretion should be measured in this setting, particularly if other signs of the nephrotic syndrome (such as edema and hyperlipidemia) are absent. The results with the dipstick and SSA serve as only a rough guide of the degree of protein excretion since urine concentration will affect the measurement. Determination of the degree of protein excretion is a central part of the evaluation of patients with acute and chronic kidney diseases The degree of proteinuria is prognostically important in patients with a primary glomerular disease. Most patients with the benign forms of isolated proteinuria excrete less than 1 to 2 g/day. The degree of proteinuria is used to monitor the response to therapy. Most patients with persistent proteinuria should undergo a quantitative measurement of protein excretion. 24-hour urine measurement; however, collecting these specimens is cumbersome in ambulatory care settings. Random urine specimen to estimate the degree of proteinuria .This test calculates the total protein-to-creatinine ratio (mg/g). This ratio correlates with daily protein excretion expressed in terms of g per 1.73m2 of body surface area . It is important to note the units of measurement in your laboratory. If the urinary creatinine concentration is measured in mmol/L, the formula must be amended as follows, since 1 mg/dL equals 0.088 mmol/L: Protein excretion = (Urine [protein] x 0.088) ÷ Urine [creatinine] Microalbuminuria is defined as persistent albumin excretion between 30 and 300 mg/day (20 to 200 µg/min). The normal rate of albumin excretion is less than 20 mg/day (15 µg/min). The standard urine dipstick is an insensitive method to detect microalbuminuria, which is the earliest clinical manifestation of diabetic nephropathy and, in patients without diabetes, is a marker of increased cardiovascular risk. Dipsticks are available that detect the urine albumin concentration in this range, but the preferred test for diagnosis and monitoring is the urine albumin-tocreatinine ratio. The urine sediment should be examined for other signs of glomerular disease such as hematuria, red cell casts, or lipiduria and Red cell casts. If the sediment is unremarkable, the differential diagnosis includes transient proteinuria, orthostatic proteinuria, and persistent proteinuria. The urine dipstick should be repeated on at least one other visit. If these subsequent tests are negative for protein, the likely diagnosis is transient proteinuria. Transient proteinuria is common: (fever and exercise) With marked exercise, the excretion of both albumin and low molecular weight proteins is increased, suggesting both an increase in glomerular permeability (to allow the filtration of albumin) and a reduction in proximal reabsorption. If the patient is younger than age 30 and has documented proteinuria on more than one occasion. This test detects orthostatic proteinuria, a relatively common finding in adolescents (occurring in 2 to 5 percent), but an uncommon disorder in those over the age of 30 . Orthostatic proteinuria is characterized by increased protein excretion in the upright position, but normal protein excretion when the patient is supine ?neurohumoral activation and altered glomerular hemodynamics Total protein excretion is generally less than 1 g/day in orthostatic proteinuria, but may exceed 3 g/day in selected patients. Orthostatic proteinuria is a benign condition requiring no further evaluation or specific therapy .In many patients, the condition resolves over time. Many patients with glomerular disease will have a modest reduction in protein excretion when supine. However, the diagnosis of orthostatic proteinuria requires that protein excretion be normal when supine (less than 50 mg per 8 hours), not merely less than when in the upright position Split urines are collected according to the following protocol . o A 16-hour upright collection is obtained between 7 AM and 11 PM, with the patient performing normal activities and finishing the collection by voiding just before 11 PM. (The times can be adjusted according to the normal times at which the patient awakens and goes to sleep.) o The patient should assume the recumbent position 2 hours before the daytime collection is finished to avoid contamination of the supine collection with urine formed when in the upright position. o A separate overnight 8 hour collection is obtained between 11 PM and 7 AM. Instead of the cumbersome 24-hour urine collection, the protein-to-creatinine (Pr/Cr) ratio may be used on a first morning spot urine specimen and on a specimen collected while upright. For this, the patient is instructed to void before going to bed and to remain recumbent until the first morning sample is obtained. A thorough evaluation Underlying renal or systemic disorder . Renal function tests :BUN and Cr ,quantitative measurement of urine protein, ultrasound examination All patients with persistent proteinuria should be referred to a nephrologist for decisions regarding further management (eg, renal biopsy). A renal biopsy is performed if there is some sign of severe or progressive disease, such as nephrotic syndrome, increasing protein excretion, or an elevation in the plasma creatinine concentration. Biopsies are often not performed among patients with stable non-nephrotic proteinuria, providing renal function is stable and hematuria is not present, since knowledge of histology obtained by the biopsy is unlikely to alter therapy. The level of non-nephrotic proteinuria that should be evaluated by biopsy is not clear. Perform a biopsy in patients with non-nephrotic proteinuria of 2 to 3 g/day but not for proteinuira that is less than 1 g/day .If a patient with proteinuria greater than 1 g/day is reluctant to undergo biopsy, absolute indications include increasing proteinuria or plasma creatinine concentration, or a significant elevation in blood pressure over baseline values. The prognosis of patients with glomerular proteinuria is related to the quantity of protein excreted. Non-nephrotic proteinuria (less than 3 g/day) is associated with a much lower risk of progressive chronic kidney disease than nephrotic range proteinuria. These observations indicate the need for persistent monitoring of patients with apparently benign, nonorthostatic, and persistent isolated proteinuria. Is not a disease but a group of signs and symptoms seen in patients with heavy proteinuria Presents with edema proteinuria usually > 3.5g / 24hrs (>0.05g / kg / 24hrs in children) serum albumin < 30g/L other features: hyperlipidaemia, and hypercoaguable state Proteinuria > 3.5 grams/day Hypoalbuminemia Edema Lipiduria Hyperlipidemia Proteinuria: due to an increase in glomerular permeability Hypoalbuminemia: occurs when liver synthesis cannot keep up with urine losses Edema mechanism is complex and still in dispute: primary salt and water retention associated with reduced renal function as well as reduced plasma oncotic pressure are primary factors (overfill and underfill) Minimal change disease fits the underfill theory best Hyperlipidemia: increased liver synthesis Hypercoagulation: increased fibrinogen and loss of antithrombin III Primary Membranous Glomerulopathy Focal Segmental Glomerulosclerosis Minimal Change Disease Membranoproliferative GN Secondary Diabetic glomerulosclerosis Paraproteinemia /Amyloidosis Lupus Nephritis Systemic diseases diabetes mellitus amyloidosis SLE and other connective tissue diseases HIV/Aids nephrotoxins nsaids mercury poisoning penicillamine gold salts Allergies bee sting pollens poison ivy Circulatory effects congestive cardiac failure constrictive pericarditis renal vein thrombosis (cause or result?) Neoplastic leukemia solid tumors Nephrotic syndrome Nephrotic range proteinuria – adults >3.0-3.5 grams/d – children >40 mg/h Defining Clinical features – Proteinuria causes Hypoalbuminemia – Edema - altered Starling forces – Hyperlipidemia - increased liver production of beta lipoprotein, decreased lipoprotein lipase, decreased conversion of Free fatty acid to triglycerides Nephrotic syndrome Hypercoagulable – urinary loss of anti-thrombin III and plasminogen – hemoconcentration Risk of Infections -urinary loss of gamma globulin History and Physical Exam Urine Analysis Lab Studies Renal Biopsy Urine Sediment Types Nephritic urine red cells (hematuria) Nephrotic urine heavy proteinuria Chronic GN proteinuria variable proteinuria free fat droplets granular casts fatty casts variable hematuria broad waxy casts 24 urine collection Serum electrolytes, BUN, creatinine, lipid profile, serum albumin Serological work up - depends upon the clinical presentation - common serological tests done are: ANA; Anti-ds DNA Complement levels (C3, C4) Hepatitis B and C serologies SPEP; UPEP, ANCA, anti GBM Abs Renal Ultrasound Renal Biopsy Na+< 60 mmol/24 hrs water restriction diuretics (if not volume depleted) reduced protein diet (controversial) treat infections prophylaxis for thrombosis specific therapy corticosteroids immunosuppression Edema - salt restriction, diuretics Hyperlipidemia - lipid lowering agents ACE/ARB decrease Pgc and decrease proteinuria Renal protective Hypercoagulable - ASA Specific treatment based on biopsy Minimal change disease prednisone for 16 weeks (p) prednisone and cyclophosphamide ( p+c) FSGS p, p+c, p+cyclosporine(cs) Membranous Ponticelli Regimen p+c Most common in children Etiology: Idiopathic Drugs – NSAID Toxins – Mercury, Lead Infections – HIV, Mononucleosis Tumors – Hodgkin disease Patient usually normotensive, nephrotic sediment, normal renal function. Pathogenesis - unknown, most likely autoimmune Treatment 85% respond to steroids If resistant to steroid may require cyclophosphamide Most common primary renal disease in African-Americans Etiology: Idiopathic Drugs – Intravenous heroin Infections – HIV Others – reflux nephropathy, obesity Patient usually hypertensive. Usually progresses to ESRD over 5-20 years. Pathogenesis Primary - immunologic? Secondary - common end point of many renal diseases Treatment Steroids Immunomodulators - cyclosporine, Tacrolimus, mycophenolic acid ACE/ARB and anti-lipid agents Most common cause of idiopathic nephrotic syndrome in Caucasian adults. Heavy proteinuria is common. Hypertension and azotemia develops as disease progresses. Increased incidence of renal vein thrombosis. Primary Autoantibody to glomerular antigen Secondary Circulating antigen deposits Antibody to antigen attaches causing immune reaction In both Complement activated and cell damage due to C5-9 attack complex Rule of 1/3 1/3 – Spontaneous remission 1/3 – Partial remission / slow deterioration 1/3 – Progress to ESRD Steroids alone are not very effective Methylprednisolone alternating with chlorambucil, Methylprednisolone alternating with cyclophosphamide. Cyclosporin 1-Declining renal function 2- Persistent proteinuria 3-Amount of proteinuria at the time of presentation 4-male sex 5-Advanced age (>50) 6-Poorly controlled HTN 7-Crescents on renal biopsy 8-The presence of frequent mononuclear cells in the interstitium 9-FSGS superimposed on membranous glomerulopathy Most common cause of nephrotic syndrome in adults. Leading cause of ESRD in USA 30% of patients with Type I and 20% of patients with Type II DM develop diabetic nephropathy. Initially microalbuminuria followed by heavy proteinuria and decline in renal function. Diagnosis usually made on clinical grounds and biopsy not needed. Glomerular Hyperfiltration Efferent arteriolar vasoconstriction Afferent arteriolar vasodilation Hyperglycemia Advanced glycation endproducts Glycation of proteins Blood glucose control Control of hypertension Use of ACE inhibitors/ARBs Control of hyperlipidemia Smoking cessation Protein Restriction Myeloma Cast Nephropathy Disease of older adults Usually presents with ▪ Bone pain ▪ Hypercalcemia ▪ Pathologic fractures ▪ anemia ▪ Proteinuria - consists of monoclonal immunoglobulins Myeloma Cast Nephropathy Path. shows multiple intraluminal proteinaceous casts Pathogenesis ▪ Disorder of plasma cells with overproduction of the antibody light chain Treatment ▪ Hydration, plasmapheresis to remove the abnormal protein and chemotherapy to suppress plasma cells Primary Amyloid Amyloid deposition in heart, GI Tract, Skin, nerves and tongue No bone lesions or excess marrow plasma cells Secondary amyloid Chronic Inflammatory conditions Tuberculosis, Chronic osteomyelitis, Rheumatoid arthritis, ulcerative colitis, Hodgkins Disease Amyloid protein produced in plasma cell dyscrasias is preferentially lamda light chains Kappa chain can occur and termed light chain deposition disease Kappa chain deposits tend to deposit in tubules and glomeruli, spare vessels Kappa more aggressive than lambda Hematuria that is not explained by an obvious underlying condition is fairly common. In many such patients, particularly young adult patients, the hematuria is transient and of no consequence .On the other hand, there is an appreciable risk of malignancy in older patients (eg, over age 40) with hematuria, even if transient .However, even among older patients, a urologic cause for the hematuria can often not be identified . Gross hematuria is suspected because of the presence of red or brown urine. The color change does not necessarily reflect the degree of blood loss, since as little as 1 mL of blood per liter of urine can induce a visible color change. Gross hematuria with passage of clots almost always indicates a lower urinary tract source. As contamination with blood is a possibility in menstruating and post-partum women, urine for analysis is best obtained when the other cause of bleeding has ceased. If this is not possible a tampon can be inserted, and urinalysis obtained after the perineum is thoroughly cleansed. The initial step in the evaluation of patients with red urine is centrifugation of the specimen to see if the red or brown color is in the urine sediment or the supernatant. Hematuria is responsible if the red to brown color is seen only in the urine sediment, with the supernatant being clear. If it is the supernatant that is red to brown, then the supernatant should be tested for heme (hemoglobin or myoglobin) with a urine dipstick. A red to brown supernatant that is negative for heme (hemoglobin or myoglobin) is a rare finding that can be seen in several conditions, including porphyria ,the use of the bladder analgesic phenazopyridine, and the ingestion of beets in susceptible subjects. Medications: Doxorubicin, Chloroquine Deferoxamine, Ibuprofen ,Iron, sorbitol, Nitrofurantoin ,Phenazopyridine, Phenolphthalein, Rifampin. Food dyes :Beets (in selected patients), Blackberries, Food coloring. Metabolities: Bile pigments, Homogentisic acid, Melanin, Methemoglobin, Porphyrin , Tyrosinosis, Urates. Microscopic hematuria may be discovered by accident when blood (either red blood cells or hemoglobin) is found on a urinalysis or dipstick done for other purposes. Although abnormal hematuria is commonly defined as the presence of more than 2 RBCs per high power field in a spun urine sediment, there is no "safe" lower limit below which significant disease can be excluded . Some experienced clinicians prefer to define hematuria by quantitative counting of RBCs in a hemocytometer chamber. A count of more than 8000 per mL in centrifuged urine (with back calculation to the original volume of the urine sample) or 13,000 per mL in uncentrifuged urine is considered abnormal . The urine sediment (or direct counting of RBC per mL of uncentrifuged urine) is the gold standard for the detection of microscopic hematuria. Dipsticks for heme detect 1 to 2 RBCs per high power field and are therefore at least as sensitive as urine sediment examination, but result in more false positive tests due to the following: Semen is present in the urine after ejaculation and may cause a positive heme reaction on the dipstick . An alkaline urine with a pH greater than 9 or contamination with oxidizing agents used to clean the perineum. The presence of myoglobinuria. positive dipstick test must always be confirmed with microscopic examination of the urine The identification of the glomeruli as the source of bleeding is important both prognostically and to optimize the subsequent evaluation. In particular, patients with clear evidence of glomerular hematuria do not need to be evaluated for potentially serious urologic disease . Glomerular hematuria may result from immunemediated injury to the glomerular capillary wall, or in noninflammatory glomerulopathies such as thin basement membrane nephropathy from localized gaps in the glomerular capillary wall. signs of glomerular bleeding (best identified by a nephrologist or other experienced examiner) include red cell casts (considered by most to be pathognomonic for glomerular disease, although rarely described in acute interstitial nephritis), protein excretion exceeding 500 mg/day at a time when there is no gross bleeding, a dysmorphic appearance of some red cells (particularly acanthocytes, which are best seen by phase contrast microscopy), and brown, cola-colored urine with gross hematuria. An additional important abnormality that may be seen in patients with hematuria is blood clots. Clots virtually never occur in glomerular disease, perhaps due to the presence of urokinase and tissue-type plasminogen activators in the glomeruli and the renal tubules. As a general principle, hematuria itself is not dangerous unless extraglomerular bleeding is so brisk that it causes clots that obstruct the ureter(s). The evaluation should address the following three questions: Are there any clues from the history or physical examination that suggest a particular diagnosis? Does the hematuria represent glomerular or extraglomerular bleeding? Is the hematuria transient or persistent? Concurrent pyuria and dysuria, which are usually indicative of a urinary tract infection, but may also occur with bladder malignancy. A recent upper respiratory infection raises the possibility of postinfectious glomerulonephritis or IgA nephropathy. A positive family history of renal disease, as in hereditary nephritis, polycystic kidney disease, or sickle cell disease. Unilateral flank pain, which may radiate to the groin, suggests ureteral obstruction due to a calculus or blood clot, but can occasionally be seen with malignancy. Symptoms of prostatic obstruction in older men such as hesitancy and dribbling. there is general agreement that the presence of BPH should not dissuade the clinician from pursuing further evaluation of hematuria, particularly since older men are more likely to have more serious disorders such as cancer of the prostate or bladder. Among those with gross hematuria in whom no other cause can be identified, finasteride usually suppresses the hematuria. Recent vigorous exercise or trauma. History of a bleeding disorder or bleeding from multiple sites due to excessive anticoagulant therapy. Cyclic hematuria in women that is most prominent during and shortly after menstruation, suggesting endometriosis of the urinary tract . Contamination with menstrual blood is always a possibility, and should be ruled out by repeating the urinalysis when menstruation has ceased. Medications that might cause nephritis (usually with other findings, typically with renal insufficiency). Black patients should be screened for sickle cell trait or disease, which can lead to papillary necrosis and hematuria. Travel or residence in areas endemic for Schistosoma haematobium or tuberculosis. Sterile pyuria with hematuria, which may occur with renal tuberculosis, analgesic nephropathy and other interstitial diseases. The main indication for performing a renal biopsy in patients with isolated glomerular hematuria is evidence of progressive disease as manifested by increasing protein excretion and/or an elevation in the serum creatinine concentration Renal biopsy is not usually performed for isolated glomerular hematuria (ie, no proteinuria and no elevation in serum creatinine), since there is no specific therapy for these conditions and the renal prognosis is excellent as long as there is no proteinuria or elevation in serum creatinine. There is no cause of low-level hematuria that, in the absence of other signs and symptoms, requires immediate diagnosis. As a result, it is reasonable to repeat an abnormal urinalysis in a few days to determine if hematuria is transient or persistent. Transient microscopic hematuria is a common problem in adults. No obvious etiology can be identified in most patients with transient hematuria. Fever, infection, trauma, and exercise are potential causes of transient hematuria. Transient hematuria can also occur with urinary tract infection An important exception to the typically benign nature of transient hematuria occurs in patients over age 40 in whom even transient hematuria carries an increased risk of malignancy All patients should have a urine culture to exclude infection prior to evaluation of hematuria. The urinalysis should be rechecked in six weeks to determine whether hematuria has resolved. Patients who have resolution of hematuria do not require further evaluation. A voided urine specimen should be sent for cytology in patients at increased risk for urothelial cancers. The sensitivity of urine cytology is greatest for carcinoma of the bladder (approximately 90 percent). By comparison, sensitivity for upper tract transitional cell carcinoma is limited.Urine cytology is frequently obtained during cystoscopy, which is usually performed in patients at risk for malignancy. Among patients at low risk for genitourinary cancer, some investigators recommend that either urine cytology or cystoscopy be performed .Cystoscopy is subsequently done if malignant and/or atypical/suspicious cells are identified. Once glomerular bleeding has been excluded in a patient with otherwise unexplained hematuria, the diagnostic work-up should include a search for lesions in the kidney, collecting system, ureters, and bladder. The diagnostic yield in adults increases with age and may be higher for gross hematuria than for microscopic hematuria. The optimal radiologic evaluation for isolated hematuria is uncertain A number of modalities are available to evaluate the genitourinary tract among patients with unexplained hematuria. These include conventional radiography, IVP, retrograde pyelography, ultrasonography,MRI,MRU, conventional CT scanning, and multidetector CT urography. Patients with malignancy in the bladder or kidneys are at increased risk for malignancy at the other site and should undergo further evaluation. Screening for hematuria in patients who have no symptoms suggestive of urinary tract disease is not recommended. Polyuria has generally been defined as a urine output exceeding 3 L/day in adults. It must be differentiated from the more common complaints of frequency or nocturia, which are not associated with an increase in the total urine output. In the absence of a glucose-induced osmotic diuresis in uncontrolled diabetes mellitus, there are three major causes of polyuria in the outpatient setting, each of which is due to a defect in water balance leading to the excretion of large volumes of dilute urine (urine osmolality usually below 250 mosmol/kg): primary polydipsia, which is primarily seen in adults and adolescents; central DI; and nephrogenic DI . Primary polydipsia also called psychogenic polydipsia :a primary increase in water intake. This disorder is most often seen in anxious, middle-aged women and in patients with psychiatric illnesses, including those taking a phenothiazine which can lead to the sensation of a dry mouth. Primary polydipsia can also be induced by hypothalamic lesions that directly affect the thirst center, as may occur with an infiltrative disease such as sarcoidosis. Central DI also called neurohypophyseal or neurogenic DI is associated with deficient secretion of ADH. This condition is most often idiopathic (possibly due to autoimmune injury to the ADH-producing cells), or can be induced by trauma, pituitary surgery, or hypoxic or ischemic encephalopathy. Rare familial cases have been described. Nephrogenic DI is characterized by normal ADH secretion but varying degrees of renal resistance to its water-retaining effect. This problem, in its mild form, is relatively common, since most patients who are elderly or who have underlying renal disease have a reduction in maximum concentrating ability. This defect, however, is not severe enough to produce a symptomatic increase in urine output. Symptomatic polyuria due to ADH-resistance is seen primarily in 2 settings : Nephrogenic DI presenting in childhood is almost always due to inherited defects. Nephrogenic DI presenting in adults is almost always acquired with chronic lithium use and hypercalcemia being the most common causes of a defect severe enough to produce polyuria. The cause of polyuria is often suggested from the history (eg, age of onset and eliciting the possible presence of the different causes of DI), and rarely by the plasma sodium concentration. Specific testing is then performed to establish the diagnosis. Onset of polyuria : In the majority of cases of hereditary nephrogenic DI, severe polyuria manifests during the first week of life. In familial central DI (usually an autosomal dominant disease), polyuria may present after the first year of life, sometimes in young adulthood, due to preservation of function of the normal allele. In adults, the onset is usually abrupt in central DI ("I suddenly began urinating too much a few days ago"), and almost always gradual in acquired nephrogenic DI or primary polydipsia. The new onset of nocturia in the absence of other causes of nocturia is often a first clue to DI. The urine is normally most concentrated in the morning due to lack of fluid ingestion overnight; as a result, the first manifestation of a loss of concentrating ability is often nocturia. Family history : There are familial forms of both central and nephrogenic DI. A family history of polyuria is helpful both for diagnosis and to identify asymptomatic members of affected families who harbor the suspect allele. Thus, all families with hereditary DI should have their molecular defect identified. Plasma sodium and urine osmolality: Each of the three causes of polyuria is associated with an increase in water output and the excretion of a relatively dilute urine. With primary polydipsia, the polyuria is an appropriate response to enhanced water intake; in comparison, the water loss is inappropriate with either form of DI. Measurement of the plasma sodium concentration and the urine osmolality may be helpful in distinguishing between these disorders: A low plasma sodium concentration (less than 137 meq/L) with a low urine osmolality (eg, less than one-half the plasma osmolality) is usually indicative of water overload due to primary polydipsia. A high-normal plasma sodium concentration (greater than 142 meq/L, due to water loss) points toward DI, particularly if the urine osmolality is less than the plasma osmolality . A normal plasma sodium concentration is not helpful in diagnosis but, if associated with a urine osmolality more than 600 mosmol/kg, excludes a diagnosis of DI. Measurement of urine output : Potential problems include an incomplete collection and the necessity for multiple containers if the patient has severe polyuria, which can be minimized by obtaining an eight hour rather than a 24 hour collection. Measurement of urinary creatinine excretion can help determine if the collection is complete. Water restriction test : Even if the history and/or plasma sodium concentration and urine osmolality appear to be helpful, the diagnosis should be confirmed by raising the plasma osmolality either by water restriction .Water restriction is important to differentiate central DI from primary polydipsia; simply giving exogenous ADH. Plasma and urine ADH measurement — Unless the history and water restriction test provide unequivocal results SOLUTE DIURESIS: Glucosuria is the major cause of an osmotic diuresis in outpatients, but other conditions are often responsible when polyuria develops in the hospital. These include high-protein feedings (in which urea acts as the osmotic agent) and volume expansion due to saline loading or the release of bilateral urinary tract obstruction. A solute diuresis can be differentiated from diabetes insipidus based upon the following findings: The urine osmolality Total solute excretion



![Detection of proteinuria[1]](http://s3.studylib.net/store/data/007549979_2-02bd2c299a632d6a55125f2f2a73449c-300x300.png)


