Banded Iron Formation
advertisement

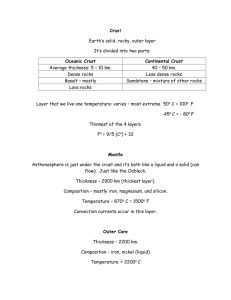
Archean Greenstone Belt 1. Greenstone Belt Greenstone belts are generally elongate, Archean to Proterozoic terrains comprising intrusive and extrusive mafic to ultramafic igneous rocks, felsic volcanics, and inter-flow or cover sedimentary rocks. Greenstone belts occur sandwiched between regions dominated by granitoids and gneiss. Greenstones are generally of low to moderate metamorphic grade. The term greenstone comes from the green color of many mafic to ultramafic constituents due to an abundance of chlorite. A common igneous rock in greenstones is komatiite. Komatiites are rocks with greater than 18 weight percent magnesium oxide and a well-developed spinifex texture of inter-locking bladed or acicular crystals of olivine or pyroxene. Spinifex texture (named after similarities in crystal shape and pattern to the spinifex grass that grows in South Africa and Western Australia) implies rapid cooling or decompression of the magma. Komatiites formed as volcanic flows and less commonly as intrusive sills. Sedimentary sequences within greenstone belts comprise both clastic (e.g., conglomerate, quartz arenite, shale and graywacke) and chemically precipitated (e.g., banded iron formation and chert) components. Greenstones may also be intruded by syn-to post-tectonic granitoids. Greenstone belts host many major mineral deposits, such as gold and nickel. Greenstone belts were previously often thought to continue to large depths in the crust. Reflection seismic profiles over the Norseman Wiluna Belt of the Yilgarn Craton, Western Australia, however, indicate that this greenstone belt has a relatively shallow (6–9 km) flat-base and overlies a uniformly thick crust. Contrasting models have been proposed for the origins of greenstone belts. Some geologists believe magmatic and tectonic processes during formation of greenstone belts in Archean times were different to presentday plate tectonics. Earth's mantle would have then been far hotter. They cite differences between greenstone belts and Phanerozoic orogens (such as the abundance of komatiitic lavas) and point out that there are no modern analogues to greenstone belts. Opponents to Archean plate tectonics contend that greenstone belts commonly represent a laterally continuous volcano sedimentary sequence (sometimes on a granite-gneiss basement) essentially undeformed prior to late tectonism and may not therefore represent relics of volcanic chains. They consider that Archean tectonics was dominated by mantle plumes and was possibly analogous to the tectonics of Venus. Greenstone belts are interpreted as oceanic plateaus generated by mantle plumes, similar to plume-generated oceanic plateaus in the southern Caribbean. A mantle plume origin is also proposed for neighboring tonalite-trondhjemite-granodiorite sequences. The alternate view is that tectonic processes comparable to presentday plate tectonics were operative during the Late Archean, and possibly were similar to plate tectonics since the Hadean-Archean transition (between 4.0 and 4.2 billion years ago). In a plate tectonic context, greenstones may have formed in volcanic arcs or inter-arc or back-arc basins. Greenstone belts are interpreted to represent collages of oceanic crust, island arcs, accretionary prisms, and possible plateaus. Recent experimental work on the origin of komatiitic magmas indicates that they were hydrous and that temperatures for their formation do not indicate that the Archean upper mantle was significantly hotter than today. Komatiites and similar rocks have also been found in younger orogens. Komatiites may not therefore require different tectonic processes or conditions for their formation, as previously thought. Isua greenstone belt (Greenland) The Isua Greenstone Belt is an Archean greenstone belt in SW Greenland dated at 3.8-3.7 Ga and contains the oldest known, well preserved, metavolcanic (metamorphosed mafic volcanic), metasedimentary and sedimentary rocks on Earth. Almost all the rocks are deformed and substantially altered by metasomatism, however the transitional stages from the volcanic and sedimentary structures to schists can clearly be seen. New geological mapping studies are tracing the transitional gradations between the protoliths and their diverse deformed and metasomatized structures. These new mappings show that most of the Isua Greenstone Belt consists of fault bounded rock assemblies (1) derived from basalt and high-Mg basaltic pillow lava, (2) intruded by numerous sheets of tonalite, (3) intercalated with chert-banded iron formations, and a minor component of clastic sedimentary rocks derived from chert and basaltic volcanic rocks. It is thought that the recrystallized ultramafic bodies that occur in the belt are intrusions or komatiitic flows. Studies show that these komatiites are extremely similar to the 3.5 Ga Barberton basaltic komatiites of South Africa, and are both Archean equivalents of modern boninites produced by hydrous melting in subduction zones. The Barberton komatiites share some of the same geochemical characteristics with modern-day boninites, including petrologic evidence for high magmatic water content. The boninitic geochemical signatures provide evidence that plate tectonic processes are responsible for the creation of the belt, and that the pillow breccias and basaltic debris indicate that liquid water existed on the surface at the time of their formation. The most common sedimentary rocks are the chert banded iron formations. Belingwe Greenstone Belt (Zimbabwe) Samples taken from the NERCMAR drill hole in the 2.7 Ga Manjeri Formation in the Belingwe Greenstone Belt contain oxide and sulphide ironstones that are indicative of a complex bacteria community. The REE compositions imply an oceanic deposition similar to that of the late Archean (Bickle et. al, 1999). The Belingwe Greenstone Belt contains a 7 km succession of mafic and ultramafic lavas and high-level intrusions which overlie a thin sedimentary formation, itself unconformable on a granitic basement. The lavas range in composition from andesites (4 per cent MgO) to peridotitic komatiites (32 per cent MgO). The mineralogy and textures of the most magnesian lavas demonstrate that they were extruded in a completely liquid state. If the source mantle had an MgO content around 40 per cent, then partial melts in the range 35 per cent to 55 per cent would be required to produce the most magnesian liquids observed. Physical constrains on the origin of the mafic and ultrafic lavas Imply a derivation from a depth of >150 km, at temperatures of 1600-2000 oC (Nisbet et al., 1977). Greenstone Belts in the Superior Province and the Evolution of Archean Tectonic Processes (Development of Abitibi Greenstone Belt) The origin and development of Archean greenstone belts continues to be strongly debated, particularly with regard to the roles of subduction, plume magmatism, rifting, diapirism and autochthonous vs allochthonous development (e.g. de Wit, 1998; Hamilton, 2003). It is apparent from studies in the Superior and Slave Provinces of Canada that strongly contrasting tectonic styles may have been in operation at the same time. For example at ca. 2.7 Ga, large diapiric batholiths and synclinal greenstone keels may suggest that diapirism was an important tectonic process in the Slave Province (Bleeker, 2002), whereas in the Superior Province the linear distribution of belts suggests that accretionary tectonics (i.e. plate tectonics) may have dominated (e.g. Stott, 1997). Neither theory precludes the other, and in developing models for Archean tectonic evolution, no one model will be equally applicable to all areas. Greenstone belt types 1) Flood volcanism on submerged (shallow water) continental platforms – these sequences contain thick, laterally extensive tholeiitic mafic flows, pillow basalts, hyaloclastite ± komatiites and minor amounts of felsic tuff, BIF, cherty and clastic sedimentary units. Rarely unconformities are preserved at the base of these sequence, where thin conglomerate-quartzite-arkose-carbonate units overly tonalitic basement. More commonly the base of the sequence is not preserved but detrital zircon ages in sedimentary rocks correspond to the age of nearby granitoid rocks which are inferred to represent basement. The volcanic rocks may also contain xenocrystic zircons, Nd isotopic evidence of older crustal involvement, and geochemical signatures suggesting felsic crustal assimilation. These sequences typically occur early in the development of Archean cratons and are common at 3.0-2.9 Ga in the Superior Province (North Caribou and Marmion terranes). In the North Caribou terrane evidence of graben development exists in the basement suggesting extension prior to volcanism, and plume-driven rifting has been suggested as the environment of formation. This environment does not have a true modern analogue as the continental flood basalts on the modern Earth are subaerial. 2) Submarine volcanic plains – comprise massive and pillowed tholeiitic mafic flows ± komatiite, BIF, mudstones and rare greywackes. The lavas have juvenile Nd isotopic signatures, lack significantly older zircon inheritance and have primitive mantle normalised REE profiles that are flat to slightly depleted in light REE (but less depleted than modern MORB) or slightly enriched in light REE. The sequences often have tectonic contacts and may be fragments of thicker sequences. They appear to have formed in oceanic environments, and suggested tectonic settings are oceanic plateau, back-arc basin, primitive island arc, oceanic island or possibly mid-ocean ridge. These sequences often flank older continental blocks and may have been accreted to continental margins. Examples are the older (ca. 2.88 Ga?) parts of the OxfordStull Lake terrane flanking the northern margin of the North Caribou terrane; the older (ca. 2.78 Ga) parts of the Western Wabigoon terrane flanking the Winnipeg River and Marmion terranes; and parts of the ca. 2.75-2.70 Ga Abitibi subprovince. 3) Diverse volcanic sequences – comprise a variety of submarine to lesser subaerial units, dominated by basalt, with lesser andesite, dacite and rhyolite flows, and dacite-rhyolite pyroclastic units. In some belts komatiites are also present. Both tholeiitic and calcalkaline signatures are often observed, with basalts ranging from slightly light REE depleted (but less depleted than modern MORB) to moderately light REE enriched with negative Nb anomalies (similar to modern arc related basalts). These sequences are commonly associated with synvolcanic granitoids. Nd isotopic data suggest that some sequences are juvenile while others have experienced minor interaction with older enriched sources (metasomatised mantle or crustal contamination?). They are suggested to represent arc magmatism (both island arc and continental arc on a thin margin), arc/plume interaction, arc rifting or back-arc magmatism. Examples are 2.83 Ga sequences in the OxfordStull Lake terrane flanking the northern margin of the North Caribou terrane; various ca. 2.9-2.74 Ga sequences in the Uchi subprovince at the southern margin of the North Caribou terrane; 2.75-2.71 Ga sequences in the Western Wabigoon terrane; and parts of the ca. 2.75-2.70 Ga Abitibi subprovince 4) Continental felsic volcanic centres – comprise thick sequences of massive calc-alkaline dacite-rhyolite flows and pyroclastics with lesser calc-alkaline basalts and andesites and syn-volcanic plutons. They may unconformably overly older tholeiitic to calc-alkaline sequences but basal contacts are usually tectonised or intruded by younger granitoids. Indirect evidence for eruption on older basement occurs in the form of older Nd model ages, zircon inheritance, evolved geochemical signatures and the maturity of the sequences. They are suggested to represent continental arc magmatism and examples are widespread at 2.73-2.71 Ga in the western Superior Province, occurring along both the northern and southern flanks of the North Caribou terrane (in several greenstone belts along a 2000 km long continental margin), and in the eastern part of the Wabigoon subprovince. 5) Late alkaline-shoshonitic sequences – occur locally in the Superior Province associated with late transpressional faults (at ca. 2.71-2.68 Ga, younging from north to south), following the major N-S shortening event. They comprise alkaline volcanic rocks with evolved Nd isotopic signatures and geochemistry, and continent-derived alluvial-fluvial sedimentary rocks with a large diversity of detrital zircon ages. The sequences are thought to have formed in late pull-apart basins and well-developed examples include 2.71-2.70 Ga sequences along the north and south margins of the North Caribou terrane (in the Oxford-Stull Lake terrane and the Uchi subprovince); and 2.69-2.68 Ga sequences in the Abitibi subprovince where the type locality of this “Timiskaming” sequence occurs. Plate tectonics in the Archean It is clear from the Superior Province that 3.0-2.7 Ga greenstone belts formed in both oceanic and continental environments. Archean “oceanic” sequences may not represent true oceanic crust generated at mid-ocean ridges but an abundance of other oceanic environments appears to be represented by the diverse rock record (e.g. primitive island arc, back-arc and oceanic island sequences). Continental sequences also appear to represent both divergent and convergent plate settings, related to rifting or hot-spot magmatism and subduction-zone magmatism. The diversity of greenstone belt sequences requires a diversity of tectono-magmatic processes to generate them, and is most consistent with the operation of plate tectonics (or something resembling it) in the Archean. 2. Komatiite Komatiites are ultramafic mantle-derived volcanic rocks. They have low SiO2, low K2O, low Al2O3, and high to extremely high MgO. Komatiites were named for their type locality along the Komati River in South Africa. True komatiites are very rare and essentially restricted to rocks of Archean age and most are greater than two billion years old, restricted in distribution to the Archaean shield areas. Komatiites occur with other ultramafic and high-magnesian mafic volcanic rocks in Archaean greenstone belts. Komatiites are restricted to the Archean, with few Proterozoic and few Mesozoic or Phanerozoic komatiites known (although highmagnesian lamprophyres are known from the Mesozoic). This restriction in age is thought to be due to secular cooling of the mantle, which may have been up to 500 °C hotter during the early to middle Archean (4.5 to 2.6 Ga). The early Earth had much higher heat production, because of the greater abundance of radioactive elements, as elements with a relatively short half-life, such as the uranium isotope with mass 235, have appreciably diminished in abundance by radioactive decay. The youngest komatiites are from the island of Gorgona on the Caribbean oceanic plateau. Komatiite core Komatiite Thin section through the coarse-bladed olivine spinifex zone in the slowly cooled interior of a komatiite unit. Samples are ~0.5 m from upper chill margin. The long, dark stripes in the thin section outline the shapes of olivine (now altered to serpentine and magnetite) blades that reach 15 cm in length in this portion of the unit. The lighter-colored triangles and quadrilaterals contain tremolite, chlorite and magnetite and represent regions where spinel and highCa pyroxene crystallized after the early olivine blades. Diamonds in volcaniclastic komatiite from French Guiana The world's main sources of non-alluvial diamonds are found in ultrapotassic kimberlite or lamproite diatremes (pipes filled with explosive volcanic debris), most of which have Phanerozoic ages and are located in stable Precambrian cratons. Diamond exploration has therefore tended to focus on such deposits. Microdiamonds are known to occur in metamorphic rocks such as gneiss and eclogite that have equilibrated deep in the mantle and were then tectonically transported to the surface, but such deposits are thought to have little commercial potential. A new type of diamond occurrence from the Dachine region in French Guiana has been found. The host rock is volcaniclastic komatiite whose composition and origin are quite unlike those of kimberlite and lamproite. These komatiites form part of a Proterozoic island-arc sequence, a tectonic setting distinct from that of all other currently exploited diamond deposits. The discovery of diamonds in volcaniclastic komatiite has implications not only for diamond exploration, but also provides strong evidence that these komatiite magmas originated at depths of 250 km or greater within the Earth. 3. Banded iron formation (BIF) Banded iron formation is iron rich chert (cryptocrystalline silica, SiO2). The banded colors, usually on a cm scale are due to differing amounts and oxidation states of Fe-containing minerals: hematite, magnetite, grunerite, siderite and sometimes pyrite. BIF is not forming today and although it can be found in the Archean, most deposits of BIF were formed around 2 billion years ago. Banded iron formations are a distinctive type of rock often found in old sedimentary rocks. The structures consist of repeated thin layers of iron oxides, either magnetite or hematite, alternating with bands of ironpoor shale and chert. Some of the oldest known rock formations, formed around three thousand million years before present (3 Ga), include banded iron layers, and the banded layers are a common feature in sediments for much of the Earth's early history. Banded Iron Formations are composed of alternating layers of iron-rich material (commonly magnetite) and silica (chert). Each layer is relatively thin, varying in thickness from a millimeter or so up to several centimeters. BANDED IRON-FORMATIONS (BIFS) WORLDWIDE. BIFs are the diagenetic product of a chemical precipitate in a very ironrich-system: SiO2-FeO-Fe2O3-CaO-MgO-CO2-H2O. They occur in the geologic record from 3.8 Ga (Isua, West Greenland) to about 1.8 Ga with a maximal abundance at about 2.5 Ga, and a reoccurrence in Neoproterozoic time (from about 0.8 and 0.6 Ga). Many of the 3.8 to 1.8 Ga BIFs have very similar average chemistries and the late diagenetic assemblages consist mainly of chert, magnetite, hematite, stilpnomelane and carbonates (siderite, dolomite to ankerite, calcite). Regional metamorphism results in assemblages rich in various amphiboles, and at higher grades, various pyroxenes. Several major BIFs in Brazil with an age of about 2.4 Ga are much richer in Fe3+ (almost all hematite-rich) than normal, possibly as a result of deep weathering and secondary oxidation. The Neoproterozoic BIFs are chemically distinctly different from most others because about 95% of their total iron is Fe3+. 3.8 Ga Isua BIF SW Greenland Archean Folded BIF: Ord Range, W Australia Dimension: 15 cm in vertical scale Light: chert jasper bands Dark: magnetiterich bands Stromatolite, Ord Range, W Australia Stromatolite: An organosedimentary structure produced by sediment trapping, binding, and/or precipitation as a result of growth and metabolic activity of microorganism principally cyanophytes (blue/green algae). All BIFs between 3.8 and 1.8 Ga show REE patterns with pronounced positive Eu anomalies, negative Ce anomalies and depletion in the light REE. These patterns are the result of chemical precipitation from solutions that represent mixtures of seawater and hydrothermal input (of Fe and Si) from spreading centers in oceanic crust. The Neoproterozoic BIFs (e.g., Rapitan iron-formation, Yukon, Canada) display a lack of the Eu anomaly, and their overall REE pattern is very similar to that of modern ocean water at 100 m. This suggests that the hydrothermal input was highly diluted by ocean water at this late Precambrian time. The Neoproterozoic iron-formations commonly show a close association with glaciogenic deposits. The Rapitan BIF is interpreted as having been deposited during a major transgressive event with a rapid sea-level rise during an interglacial period, after earlier buildup of ferrous iron in solution in deeper water during a glacial period. Earlier banded iron formations The conventional concept is that the banded iron layers were formed in water as the result of oxygen released by photosynthetic cyanobacteria, combining with dissolved iron in Earth's oceans to form insoluble iron oxides, which precipitated out, forming a thin layer on the substrate, which may have been anoxic mud (forming shale and chert). Each band is similar to a varve. The banding is assumed to result from cyclic variations in available oxygen. It is unclear whether these banded formations were seasonal or followed some other cycle. It is assumed that initially the Earth started out with vast amounts of iron dissolved in the world's acidic seas. Eventually, as photosynthetic organisms generated oxygen, the available iron in the Earth's oceans was precipitated out as iron oxides. It is theorized that the Earth's primitive atmosphere had little or no free oxygen. In addition, Proterozoic rocks exposed at the surface had a high level of iron, which was released at the surface upon weathering. Since there wasn't any oxygen to combine with it at the surface, the iron entered the ocean as iron ions. At the same time, primitive photosynthetic blue/green algae was beginning to proliferate in the near surface waters. As the algae would produce O2 as a waste product of photosynthesis, the free oxygen would combine with the iron ions to form magnetite (Fe3O4). This cleansed the algae's environment. As the biomass expanded beyond the capacity for the available iron to neutralize the waste O2 the oxygen content of the sea water rose to toxic levels. This eventually resulted in large-scale extinction of the algae population, and led to the accumulation of an iron poor layer of silica on the sea floor. As time passed and algae populations reestablished themselves, a new iron-rich layer began to accumulate. Unfortunately, the algae would again proliferate beyond the capacity of the iron ions to clean up their waste products, and the cycle would repeat. This went on for approximately 800,000,000 years! Later banded iron formations Until fairly recently, it was assumed that the rare later banded iron deposits represent unusual conditions where oxygen was depleted locally and ironrich waters could form then come into contact with oxygenated water. An alternate explanation of these later rare deposits is undergoing much research as part of the Snowball Earth hypothesis — wherein it is believed that an early equatorial superconitent (Rodinia) was totally covered in an ice age (implying the whole planet was frozen at the surface to a depth of several kilometers) which corresponds to evidence that the earth's free oxygen may have been nearly or totally depleted during a severe ice age circa 750 to 580 Ma prior to the Ediacaran wherein the earliest multicellular lifeforms appear. Alternatively, some geochemists suggest that BIFs could form by direct oxydation of iron by autotrophic (non-photosynthetic) microbes. The total amount of oxygen locked up in the banded iron beds is estimated to be perhaps twenty times the volume of oxygen present in the modern atmosphere. Banded iron beds are an important commercial source of iron ore. BIF and oxygen state in the Archean Addition of O2 to the Atmosphere Today, the atmosphere is ~21% free oxygen. How did oxygen reach these levels in the atmosphere? Revisit the oxygen cycle: Oxygen Production 。Photochemical dissociation - breakup of water molecules by ultraviolet Produced O2 levels approx. 1-2% current levels At these levels O3 (Ozone) can form to shield Earth surface from UV 。Photosynthesis - CO2 + H2O + sunlight = organic compounds + O2 - produced by cyanobacteria, and eventually higher plants supplied the rest of O2 to atmosphere. Oxygen Consumers 。Chemical Weathering - through oxidation of surface materials (early consumer) 。Animal Respiration (much later) 。Burning of Fossil Fuels (much, much later) Cyanobacteria Throughout the Archean there was little to no free oxygen in the atmosphere (<1% of presence levels). What little was produced by cyanobacteria, was probably consumed by the weathering process. Once rocks at the surface were sufficiently oxidized, more oxygen could remain free in the atmosphere. During the Proterozoic the amount of free O2 in the atmosphere rose from 1 - 10 %. Most of this was released by cyanobacteria, which increase in abundance in the fossil record 2.3 Ga. Present levels of O2 were probably not achieved until ~400 Ma. Evidence from the Rock Record ‧Iron (Fe) is extremely reactive with oxygen. If we look at the oxidation state of Fe in the rock record, we can infer a great deal about atmospheric evolution. ‧Archean - Find occurrence of minerals that only form in non-oxidizing environments in Archean sediments: Pyrite (FeS2), Uraninite (UO2). These minerals are easily dissolved out of rocks under present atmospheric conditions. ‧Banded Iron Formation (BIF) - Deep water deposits in which layers of iron-rich minerals alternate with iron-poor layers, primarily chert. Iron minerals include iron oxide, iron carbonate, iron silicate, iron sulfide. BIF's are a major source of iron ore, they contain magnetite (Fe3O4) which has a higher iron-to-oxygen ratio than hematite. These are common in rocks 2.0 - 2.8 B.y. old, but do not form today. ‧Red beds (continental siliciclastic deposits) are never found in rocks older than 2.3 B. y., but are common during Phanerozoic time. Red beds are red because of the highly oxidized mineral hematite (Fe2O3), that probably forms secondarily by oxidation of other Fe minerals that have accumulated in the sediment. Conclusion - amount of O2 in the atmosphere has increased with time. Supplement-1 Evolution of the Hydrosphere and the Atmosphere The atmosphere and the oceans both arose from volcanic degassing very early in earth history. The lighter fraction made up the atmosphere while the heavier fraction made up the oceans. Also the presence of water served as transporter of soluble solids and gases between the land, sea and atmosphere. Early Degassing : Loss of Noble Gases Helium, argon and xenon in low concentration when compared to their cosmic abundance. It must have been lost from the earth at high rates, early on (i.e., the first 50 million years of earth history) in order to be so low now. If degassing from volcanoes has always has the same composition, the major gasses would be water vapor and CO2 while the minor gasses would be H2S, CO, H2, N2, CH4, NH3, HF, HCl, and Ar. But no oxygen. The Evolution of Oxygen Oxygen is toxic to living systems. Oxygen-mediating enzymes had to evolve to handle the toxic properties of O2 Different segments of the solar spectrum are capable of creating O2. 1. Ultra-violet (1500 and 2100 angstroms causes the photodissociation of H20 in the upper atmosphere. H2 drifts into outer space, leaving O2 behind. This this mechanism accounts for only 0.1% of the present atmospheric level i.e., PAL). 2. Visible light, used by photoautotrophs, creates far more O2 from CO2 and H2O and photosynthetic enzymes (e.g., chlorophyll). The carbon released by photosynthesis was held by these organisms by the creation of organic chemicals used in growth. Stages of Oxygen Development through Geologic Time A. Oceanic Oxygen 1. 4.0-3.2 Billion Years Ago a. Oxygen held in mantle and oceanic crust. Little outgassed b. That which did was trapped in evaporite precipitation and the oxidation of CO to CO2. c. Fe+2 and Mn+2 accumulated in the sea from hydrothermal vents and the leaching of volcanics and immature sediments. These two are soluble in their +2 state. The lack of O2 kept them reduced and soluble. 1. 3.2-2.6 Billion Years Ago Early photoautotrophic organisms release small concentrations of O2 that oxidized Fe+2 creating insoluble Fe+3 precipitates in Banded Iron Formations (i.e., BIF) in island arc (i.e., Algoma type BIF) and shallow shelf environments (i.e., Superior type BIF) as a result of oceanic upwellings. 2. 2.6-2.0 Billion Years Ago a. Development of extensive Superior-type BIF and manganese deposits capturing O2 released by the photoautotrophs. b. Stromatolites in limestones expand rapidly both providing O2 to the ocean while capturing carbon at the same time. c. The presence of oxygen cleared the sea of soluble iron and manganese so that little further oxygen was needed for BIF and manganese deposit formation. B. Atmospheric Oxygen. Oxygen produced by photoautotrophs was used in BIF and manganese deposits. Once O2 production increased beyond that needed by BIF and manganese deposit formations, could it accumulate in the ocean and in the atmosphere. Free oxygen began to accumulate in the atmosphere between 2.4 and 1.9 billion years ago. Evidence for this includes the following. 1. BIF deposits iron-leaching from paleosols disappear after 1.9 billion years. The presence of oxygen rendered iron and manganese insoluble, hence they would not leach. Leaching could only occur if iron and manganese were in the +2 state and therefore soluble and mobile. 2. Presence of detrital uraninite and pyrite 2.3 billion years ago, then disappears. Grains of uraninite and pyrite indicate they are insoluble in the absense of oxygen. Once oxygen is present, they dissolve and won't occur as solid grains (i.e., detrital). 3. Development of hematite-rich paleosols by 2.2 and 2.0 billion years indicates that there is enough oxygen in the atmosphere to oxidize iron (i.e., rust). This indicates an increase in atmospheric oxygen of 15 fold 1.9 billion years ago. 4. Appearance of CaSO4 in evaporites after 1.9 billion years ago indicates that there is enough atmospheric or oceanic oxygen to oxidize sulfides to sulfates. 5. First appearance of redbeds (red conglomerates, sandstones and shales) after 2.3 billion years ago 6. Accumulation of organic-rich limestones between 0.9 and 0.6 billion years ago indicates the retention of carbon compounds from photosynthesis which means an equal volume of oxygen had to be released into the atmosphere. The atmospheric oxygen level fluctuated during the Phanerozoic. Oxygen increased during periods when plants evolved and expanded on the continents. Oxygen decreased during arid periods, when sea levels drops because newly exposed coals and organic-rich sediments would consume oxygen in their decomposition (i.e., the opposite process of photosynthesis). Carbon Dioxide Balance CO2 concentration in the atmosphere was many hundred times greater in the Archean. This was due to a.) the oxidation of CO and CH4 to form CO2 by the ever-increasing concentrations of atmospheric and oceanic oxygen, b.) the release of CO2 during the periods of massive volcanism in the Archean and c.) the much lower rates of subduction due to the lower thermal gradients in the mantle. Higher CO2 concentrations (i.e., 80 to 600 times PAL) led to the Greenhouse effect that kept temperatures above freezing and thereby prevented glaciations. Higher CO2 concentrations led to the photochemical production of major oxidants, hydrogen peroxide (H2O2) and formaldehyde (H2CO), that enhanced ferrous iron solution in the Archean. As the CO2 concentration decreased through the Proterozoic, stromatolite production decreased. In the Phanerozoic, CO2 concentrations fluctuated for several reasons. Uplift and mountain building due to plate tectonics induces the outgassing of CO2, generated by metamorphism. CO2 concentrations drops almospheric concentrations due to loss of CO2 in the creations of deep sea and shallow water limestones (i.e., CaCO3). Burial of coal and carbonates by silicate sediments reduces CO2 concentration by burying it below the surface. So uplift increases CO2 concentration, while their erosion to low relief decreases CO2 concentration. Atmospheric Evolution and the Development of Life Reduced carbon and carbohydrate chemicals found in rocks 3.8 billion years old. First, prokarotic cell structures found around 3.5 billion years ago. Bacterial communities (stromatolites) found in rocks 3.2 billion years old. First eukarotic cells found around 1.6 billion years ago. Atmospheric oxygen levels reached about 10% PAL. Respiration, a far more efficient metabolic set up is now possible. Eukarotes mark the beginning of sexual reproduction that, in turn, means greater possibilities of genetic development. First metazoa found about 700 to 600 million years ago (0.6-0.7 billion years ago). Colligen produced and leads to the development of hardparts 570 million years ago (.57 billion years). Camrian period marks the resting removal of atmospheric CO2 from prokarotic tissue growth to metazoan eukarotic CaCO3 shell formation. Supplements-2 Types of BIF Archean Earth Banded Iron Formation (BIF) has been suggested as a possible terrestrial analog for Early Mars (Calvin, 1998). Two types of BIF in the United States and Canada have been differentiated based on their respective origins. The Algoma type deposits in Ontario, Canada are in close proximity to ancient volcanic centers suggesting a sub-aqueous hydrothermal origin similar to modern day sea-floor spreading centers (Gross, 1983). The Lake Superior type BIF deposits in the upper peninsula of Michigan are not associated with extrusive volcanic materials and are therefore interpreted as chemical precipitates of iron-rich waters in a shallow sea (James, 1954). The Thermal Emission Spectrometer (TES) discovery of crystalline, gray hematite in sedimentary basin type deposits on Mars supports the use of Lake Superior type BIF as a terrestrial analog. The Sinus Meridiani and Aram Chaos hematite sites are not in close proximity to a volcanic center, and do not exhibit any lava flow features (Christensen, et al., 2001). The Sinus Meridiani hematite occupies a smooth unit with abrupt boundaries suggesting that it exists within a stratigraphic layer. The Aram Chaos hematite appears to be within a closed basin around which outflow channels are common suggesting an aqueous origin. In both sites, the hematite appears to be part of layered, sedimentary rock units that suggest aqueous environments (Christensen, et al., 2001). The Lake Superior type BIF occurs in four principal facies: sulfide, carbonate, silicate, and oxide (James, 1954). These facies grade into each other in the field reflecting changes in the oxidation state of the water and occur as thin laminae alternating with chert layers. The mm scale laminations of these rocks will not be evident in large- scale (3km x 6km) TES spectra. The iron-rich minerals present in each facies are possible auxiliary minerals for the low albedo hematite regions on Mars. These minerals are: pyrite in the sulfide facies, siderite in the carbonate facies, minnesotaite and stilpnomelane in the silicate facies, and magnetite and hematite in the oxide facies. A field trip to the Lake Superior type deposits in the Marquette and Gogebic iron districts of Michigan has provided a thorough rock sampling of the different facies, including several types of crystalline, gray hematite. Micaceous, specular hematite with a schistose texture is highly metamorphosed and is probably not seen on the surface of Mars. Bulk, gray crystalline hematite occurs in relatively unmetamorphosed BIF and retains its sedimentary layer nature. It also displays a microplaty texture in some samples that is most likely the result of low-grade burial metamorphism. Some of the bulk, gray crystalline hematite displays magnetic properties suggesting some mixture of magnetite and hematite. The spectra of these bulk samples may be better analogs for Mars than pure mineral phases. The spectra of these samples will be presented and compared to what TES has observed. Algoma-type banded-iron formation deposit, or Algoma-type BIF deposit characteristics Algoma type was formed over a much wider time range than the Lake Superior type (from 3.8 billion to a few hundred million years ago). Algoma-type BIFs are also finely layered intercalations of silica and an iron mineral, generally hematite or magnetite, but the individual layers lack the lateral continuity of Lake Superior-type BIFs. Algoma type BIF ALGOMA-TYPE IRON FORMATION SYNONYMS: Taconite, itabirite, banded iron-formation. COMMODITIES (BYPRODUCTS): Fe (Mn). EXAMPLES: Falcon, Lady A, McLeod, Sherman, Adams, Griffith (Canada), Woodstock, Austin Brook (New Brunswick, Canada), Kudremuk (India), Cerro Bolivar (Venezuela), Carajas (Brazil), part of Krivoy Rog (Russia). GEOLOGICAL CHARACTERISTICS CAPSULE DESCRIPTION: Iron ore deposits in Algoma-type ironformations consist mainly of oxide and carbonate lithofacies that contain 20 to 40 % Fe as alternating layers and beds of micro- to macro-banded chert or quartz, magnetite, hematite, pyrite, pyrrhotite, iron carbonates, iron silicates and manganese oxide and carbonate minerals. The deposits are interbedded with volcanic rocks, greywacke, turbidite and pelitic sediments; the sequences are commonly metamorphosed. TECTONIC SETTINGS: Algoma-type iron-formations are deposited in volcanic arcs and at spreading ridges. AGE OF MINERALIZATION: They range in age from 3.2 Ga to modern protolithic facies on the seafloor and are most widely distributed and achieve the greatest thickness in Archean terranes (2.9 to 2.5 Ga). DEPOSITIONAL ENVIRONMENT / GEOLOGICAL SETTING: They formed both near and distal from extrusive centres along volcanic belts, deep fault systems and rift zones and may be present at any stage in a volcanic succession. The proportions of volcanic and clastic sedimentary rocks vary and are rarely mutually exclusive. HOST/ASSOCIATED ROCKS: Rocks associated with Algoma-type iron-formations vary greatly in composition, even within local basins, and range from felsic to mafic and ultramafic volcanic rocks, and from greywacke, black shale, argillite, and chert interlayered with pyroclastic and other volcaniclastic beds or their metamorphic equivalents. Algoma-type iron-formations and associated stratafer sediments commonly show a prolific development of different facies types within a single stratigraphic sequence. Oxide lithofacies are usually the thickest and most widely distributed units of iron-formation in a region and serve as excellent metallogenetic markers. DEPOSIT FORM: Iron ore deposits are sedimentary sequences commonly from 30 to 100 m thick, and several kilometres in strike length. In most economic deposits, isoclinal folding or thrust faulting have produced thickened sequences of iron-formation. STRUCTURE/TEXTURE: Micro-banding, bedding and penecontemporaneous deformation features of the hydroplastic sediment, such as slump folds and faults, are common, and can be recognized in many cases in strongly metamorphosed oxide lithofacies. Ore mineral distribution closely reflects primary sedimentary facies. The quality of oxide facies crude ore is greatly enhanced by metamorphism which leads to the development of coarse granular textures and discrete grain enlargement. ORE MINERALOGY: Oxide lithofacies are composed of magnetite and hematite. Some deposits consist of siderite interbedded with pyrite and pyrrhotite. GANGUE MINERALOGY (Principal and subordinate): Quartz, siderite or ferruginous ankerite and dolomite, manganoan siderite and silicate minerals. Silicate lithofacies are characterized by iron silicate minerals including grunerite, minnesotaite, hypersthene, reibeckite and stilpnomelane, associated with chlorite, sericite, amphibole, and garnet. WEATHERING: Minor oxidation of metal oxide minerals and leaching of silica, silicate and carbonate gangue. Algoma-type ironformations are protore for high-grade, direct shipping types of residual-enriched iron ore deposits. GENETIC MODEL: Algoma-type iron deposits were formed by the deposition of iron and silica in colloidal size particles by chemical and biogenic precipitation processes. Their main constituents evidently came from hydrothermal-effusive sources and were deposited in euxinic to oxidizing basin environments, in association with clastic and pelagic sediment, tuff, volcanic rocks and a variety of clay minerals. The variety of metal constituents consistently present as minor or trace elements evidently were derived from the hydrothermal plumes and basin water and adsorbed by amorphous iron and manganese oxides and smectite clay components in the protolithic sediment. Their development and distribution along volcanic belts and deepseated faults and rift systems was controlled mainly by tectonic rather than by biogenic or atmospheric factors. Sulphide facies were deposited close to the higher temperature effusive centres; iron oxide and silicate facies were intermediate, and manganese-iron facies were deposited from cooler hydrothermal vents and in areas distal from active hydrothermal discharge. Overlapping and lateral transitions of one kind of lithofacies to another appear to be common and are to be expected. ORE CONTROLS: The primary control is favourable iron-rich stratigraphic horizons with little clastic sedimentation, often near volcanic centres. Some Algoma-type ironformations contain ore deposits due to metamorphic enhancement of grain size or structural thickening of the mineralized horizon. ASSOCIATED DEPOSITS: Algoma-type iron-formations can be protore for residual-enriched iron ore deposits. Transitions from Lake Superior to Algoma-type iron-formations occur in areas where sediments extend from continental shelf to deep-water environments along craton margins as reported in the Krivoy Rog iron ranges. Oxide lithofacies of iron- formation grade laterally and vertically into manganese-rich lithofacies, and iron sulphide, polymetallic volcanic-hosted and sedex massive sulphide. ECONOMIC FACTORS GRADE AND TONNAGE: Ore bodies range in size from about 1000 to less than 100 Mt with grades ganging from 15 to 45% Fe, averaging 25% Fe. Precambrian deposits usually contain less than 2% Mn, but many Paleozoic iron-formations, such as those near Woodstock, New Brunswick, contain 10 to 40 % Mn and have Fe/Mn ratios of 40:1 to 1:50. The largest B.C. deposit, the Falcon, contains inferred reserves of 5.28 Mt grading 37.8% Fe. ECONOMIC LIMITATIONS: Usually large-tonnage open pit operations. Granular, medium to coarse- grained textures with well defined, sharp grain boundaries are desirable for the concentration and beneficiation of the crude ore. Strongly metamorphosed ironformation and magnetite lithofacies are usually preferred. Oxide facies iron-formation normally has a low content of minor elements, especially Na, K, S and As, which have deleterious effects in the processing of the ore and quality of steel produced from it. IMPORTANCE: In Canada, Algoma-type iron-formations are the second most important source of iron ore after the taconite and enriched deposits in Lake Superior-type iron-formations. Algoma-type iron-formations are widely distributed and may provide a convenient local source of iron ore. Lake Superior-type BIF deposit characteristics Sedimentary rocks deposited in the shallow waters of continental shelves or in ancient sedimentary basins. These deposits are typified by the vast BIFs around Lake Superior and are called Lake Superior-type deposits. Their individual sediment layers can be as thin as 0.5 millimetre (0.02 inch) or as thick as 2.5 centimetres (1 inch), but the alternation of a siliceous band. Spectral Properties of Lake Superior Banded Iron Formation: Application to Martian Hematite Deposits Several locations have been identified on Mars that expose bulk, coarsely crystalline gray hematite. These deposits have been interpreted as being sedimentary and formed in aqueous environments. Lake Superior Type (LST) banded iron formation (BIF) was investigated as a spectral and possible process analog to these deposits. In northern Michigan, LST BIF formed in a sedimentary, continental shelf or shallow basin environment under stable tectonic conditions, and the oxide facies contains gray, crystalline hematite. These deposits are Proterozoic in age and contain microfossils associated with the early diversification of life on Earth. Samples of the hematite-bearing oxide facies, as well as the carbonate facies, were collected and analyzed for their spectral and geochemical characteristics. Sample spectra were measured in the visible, near-infrared, and thermal infrared for comparison with remote and in situ spectra obtained at Mars. Thin section analysis, as well as X-ray diffraction and scaning electron microscopy measurements, were performed to determine detailed geochemistry. There is no evidence for BIF at Opportunity's Meridiani landing site, and the results of this work will provide useful data for determining whether BIFs exist elsewhere on Mars and are, thus, relevant to current and future Mars exploration missions. Kiruna type BIF The Fennoscandian Shield, one of the major base metal provinces in Europe, is composed of an Archean nucleus, largely unmineralized, in the northeastern part of the Shield. This nucleus is bordered to the southwest by Paleoproterozoic rocks. At c. 2.5-2.3 Ga sedimentary and volcanic rocks were deposited on the Archean basement during an extensional event. Further rifting of the continent at c. 2.1 Ga gave rise to tholeiitic and komatiitic lavas and dikes. At the end of this extensional event MORB-type pillow lavas were erupted. At c. 1.9 the tectonic regime shifted to compressive and subduction related volcanic and sedimentary rocks were deposited in a terrestrial to shallow water environment. Southwest of these intracratonic complexes, 1.95-1.87 Ga old volcanic arcs were accreted towards the craton during the Svecokarelian orogeny. This orogeny involved voluminous early calc-alkaline magmatism and ended with migmatization, S-type magmatism and large batholithic intrusions of A- to I type granitoids. Mineralization related to these Proterozoic early extensional and later comressional tectonic regimes include VMS (including Outokumpu Cu-Zn-Co±Ni type) to epithermal VMS, sedimenthosted Zn-Pb, porphyry style Cu, gold lode style deposits, BIF´s, mafic and ultramafic Ni±Cu±PGE deposits as well as Kiruna type apatite-Fe deposits, epigenetic Cu-Au deposits and syngenetic Cu deposits. The latter three types of economic deposits are included in the diverse group of Fe-oxide-Cu-Au style mineralizations: The Kiruna type apatite iron ores are hosted by 1.88 Ga felsic alkaline porphyries emplaced during compressional tectonics. The epigenetic Cu-Au deposits is a diverse group of mineralizations including vein style structurally controlled Cu-Au, probably both 1.87 Ga and 1.77 Ga in age, and intrusive hosted, porphyry style Cu-Au±Fe, related to both calc-alkaline and alkaline magmatism in a compressional regime between 1.9-1.8 Ga. The syngenetic Cu±Zn deposits restricted to the c. 2.1 Ga greenstones, formed during extensional tectonics in intracratonic rift basins. Kiruna SWEDEN (Province: Norrbotten) Long (E): 20.112, Lat (N): 67.496 Type: Apatite Fe-ore Morphology: Concordant sheet Age of mineralization: c. 1.88 Ga Ore minerals: Magnetite Alteration: albitization, actinolite, biotite-chlorite Age of host rocks: 1880±3 Ma (U-Pb), cutting dyke: 1876±9 Ma (U-Pb tit) Nature of host rocks: trachyandesite lava, felsic volcanics, intermediatemafic volcanics Cumulative past production and reserves: 2 000 Mt @ 60-68 % Fe Mineralization Ore age Palaeoproterozoic 1880 Ma Ore mineralogy Hydrothermal alteration Magnetite: Albitization and Biotitization Deposit type Magnetite-apatite deposits (tabular and pipe-like bodies, dykes) (Kiruna): Fe, P Ore shape Concordant to subconcordant mass, lens or pod of massive to submassive ore Host rock age Palaeoproterozoic 1880 ± 3 Ma U/Pb Host rock mineralogy Actinolite, Fe-Mg mica, Apatite Host rock lithology Acidic volcanic rock, Basic volcanic rock Host rock formation names Kiruna Porphyries Economy Fe Iron (metal) Past Production average Reserve: 1 200 000 000 t Average grade: 60%