Chemical Kinetics Worksheet: Reaction Rates & Mechanisms
advertisement

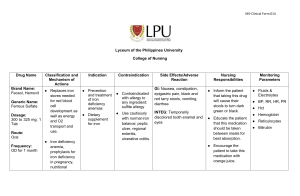
Chemical Kinetics Worksheet 1. Explain why the rate of a simple chemical reaction is likely to be most rapid at the beginning of the reaction. 2. List 4 factors which affect the rate of a homogeneous reaction. 3. What other factor will affect the rate of a heterogeneous reaction only? 4. Use collision theory to explain why reaction rates vary directly with temperature. 5. Define activated complex. 6. Draw a KINETIC ENERGY diagram for a typical exothermic reaction. 7. The following reaction has a 3 step mechanism. Given the first 2 steps, what must be the third step? Step 1) 2 NO N2O2 Step 2) N2O2 + H2 N2O + H2O Step 3) Overall) 2 NO + 2 H2 2 H2O + N2 8. If the second step for the above reaction is the slow step and the forward enthalpy change is 312 kJ, draw labelled potential energy diagrams for the forward and reverse reactions. 9. A sample iron reacted with oxygen such that the initial mass of the iron was 23.00 g and the final mass of the iron was 21.47 g. The reaction took 18.0 days according to 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) a) what is the rate in terms of grams of iron consumed per minute? b) what is the rate in terms of grams of iron(III) oxide produced per minute?