bit25813-sup-0001-SuppData
advertisement

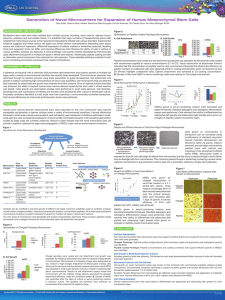
Supplementary data Inter-microcarrier transfer and phenotypic stability of stem cell-derived Schwann cells in stirred suspension bioreactor culture. Antos Shakhbazau, Leila Mirfeizi, Tylor Walsh, Holly M. Wobma, Ranjan Kumar, Bhagat Singh, Michael S. Kallos, Rajiv Midha. Supplementary Figures Supplementary Figure 1. Colonization of different carrier types (Cdx1 – Cytodex 1, Cdx3 – Cytodex 3, CspS – CultiSpher-S) by SKP-SC, %. Supplementary Figure 2. Characterization of SKP-SC in stirred 100 mL bioreactor culture on Cytodex 3 microcarriers: A,B – SKP-SC immunolabeled for GFAP, C,D SKP-SC immunolabeled for S100β (scale bars 20 µm). Supplementary Figure 3. Biodegradation of CultiSpher-S microcarriers in vivo in rat peripheral nerve at one (A), two (B) and five (C) weeks after implantation. SKP-SC grown on MC in static culture are labeled with DiI (orange). Detailed Materials and Methods Cell culture and differentiation Skin precursor cells (SKPs) were isolated and differentiated into Schwann cells according to published protocols. Briefly, skin from the dorsal torso was minced in HBSS (Gibco) on ice, incubated for 45 min in 0.1% collagenase XI at 37 °C, dissociated mechanically, washed in cold DMEM and passed through a 40 μm cell strainer. Filtrate was centrifuged at 1200 rpm and the pellet was triturated and resuspended in culture media (DMEM:F12 3:1, 100 U/mL penicillin and 100 mg/mL streptomycin, 0,25 μg/mL Fungizon, 1x B27 supplement, 20 ng/mL EGF and 40 ng/mL bFGF (all from Gibco). SKPs were cultured and passaged as undifferentiated aggregates at 37˚C in a humidified atmosphere containing 5% CO2/95% air. Progenitor state of the aggregates was confirmed with nestin immunolabeling. To induce differentiation towards Schwann cells, aggregates were triturated and replated on poly-D-lysine/laminin coated culture dishes (Corning) in SC medium - DMEM/F12 supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin, 0,25 μg/mL Fungizon, 4 μM forskolin, 10 ng/mL heregulin-1β, and 1% N2 supplement (Gibco), with 5% FBS, as described. Media were changed every 3-4 days. Following 1-2 weeks incubation, cells appearing under phase contrast to have bipolar SC morphology were isolated with cloning cylinders and their fate specification was confirmed with p75-NTR antibody staining. Culture was further enriched for p75-NTR-positive cells by means of flow cytometry-assisted sorting using flow cytometer FACSAria II (Beckton Dickinson, USA) and expanded in the SC medium until ~95% purity was achieved. These cells are subsequently referred to as SKP-derived Schwann cells, or SKP-SCs. In vitro myelination For proof-of-concept in vitro myelination assay, SKP-SC on microcarriers were seeded onto the neurite network, formed by 1 week culture of DRGs from postnatal (P10) Lewis rats in Neurobasal medium containing B27 supplement, 10% FBS, 50 ng/mL NGF, 1% penicillin/streptomycin and 0,25 μg/mL Fungizon. Assay was performed in 8 well chamber slides (Nunc) coated with Matrigel. Host SCs from DRGs were killed by the addition of 7 μM cytosine arabinoside. SKP-SCs were labeled with Cell tracker CM-DiI (Invitrogen) prior to seeding. Co-cultures were maintained in SCmedium containing 50 ng/mL NGF, and ascorbic acid (50 μg/mL, Sigma) was added to induce myelination. The co-culture was maintained for 2 weeks, then fixed with 2% paraformaldehyde in PBS, blocked/permeabilized with 2% BSA/ 0.3% Triton-X100 in PBS and stained for β-III-tubulin, myelin basic protein (MBP) and Hoechst for cell nuclei. Secondary antibodies emission spectra were 488 nm (anti-goat) for MBP or 680 nm (anti-mouse) for β-III-tubulin. Animals Animals were maintained in a temperature and humidity controlled environment with a 12-h light/dark cycle. Food (Purina, Mississauga, ON, Canada) and water were available ad libitum. Surgical interventions were carried out under inhalation anaesthetic (Isofluroane, 99.9% Halocarbon Laboratories, River Edge, NJ, USA) and pain control was provided by means of i.p. or oral administration of buprenorphine (30 μg/kg). Surgical procedures were carried out aseptically, and standard microsurgical techniques were used with an operating microscope (Wild M651; Wild Leitz, Willowdale, ON, Canada). Animals were sacrificed at endpoint under deep anaesthesia using an overdose of intracardiac Euthanol (Bimeda–MTC, Cambridge, ON, Canada). All efforts were made to minimize suffering and animal numbers by using appropriate protocols. The protocol was approved and monitored by the University of Calgary animal care committee and adhered strictly to guidelines set by the Canadian Council on Animal Care. Immunohistochemistry The regenerating cables together with adjacent nerve segments were excised, removed carefully from silicon tubes and fixed overnight in 2% paraformaldehyde in phosphate-buffered saline (PBS). Samples were washed two times in PBS, cryoprotected in 30% sucrose and embedded into optimal cutting temperature (OCT) compound (Sakura Fine technical Co., Torrance, CA, USA). Longitudinal sections (16 μm) were cut with a cryostat (Leica Microsystems Inc., Richmond Hill, ON, Canada) at 23 °C and mounted on Superfrost slides (Fisher Scientific). Sections were blocked/permeabilized with 2% BSA/ 0.3% Triton-X100 in PBS and incubated overnight at 4 °C with primary antibody anti-neurofilament NF200 (1:500, Sigma) or MBP (1:400, Santa Cruz Biotechnology). Following 3x wash with PBS, slides were incubated with secondary antibody (anti-mouse 488, Molecular Probes, or anti-goat 488, Molecular Probes) and Hoechst nuclear stain for 3 h. Slides were then washed and coverslipped using Fluorosave reagent (Calbiochem, San Diego, CA, USA) and viewed under a fluorescence microscope (Olympus BX51, Center Valley, PA, USA). Omission of primary or secondary antibody was used as negative control for the staining process.