Application Note Animal Component-Free Star-Plus Microcarriers for Adherent Mammalian Cell Culture
advertisement

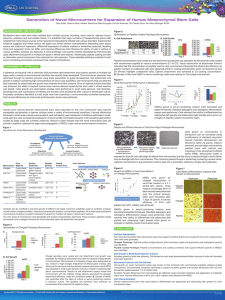
Application Note Animal Component-Free Star-Plus Microcarriers for Adherent Mammalian Cell Culture USD 3086 With the continuing move of the biopharmaceutical and cell therapy industries toward the use of animal component-free materials in biomanufacturing processes, Pall Life Sciences has added a new, animal component-free microcarrier to its already extensive portfolio. Introducing Star-Plus, a rigid, spherical, low density microcarrier, featuring a proprietary chemistry built on the SoloHill® polystyrene core resulting in one of the fastest-attaching microcarriers available for many cell types under a variety of environmental conditions. The combined features of rapid cell attachment, even cell distribution and excellent growth results in a microcarrier with key benefits for successful animal component-free cell expansion for the production of cell-based therapies and vaccine manufacturing. Like Pall’s other low density SoloHill beads, these microcarriers allow for suspension in stirred-tank reactors with gentle stirring. This enables the utilization of cells that are highly sensitive to shear stress, effectively broadening the range of adherent cell types that can be grown in suspension. The rapid attachment and even distribution of cells to these microcarriers can generate synchronous cultures which lead to increased process efficiency and consistency. In addition, these microcarriers can be gamma-irradiated in single-use biocontainers without loss of functionality, making possible a sterile, closed-system microcarrier offering that can be employed in various bioreactor formats, bypassing the need for in-house sterility validation. Materials and Methods Star-Plus Microcarriers. Star-Plus microcarriers (model# SP102-1521) were prepared and sterilized by autoclaving or gamma irradiation. Vero Cell Culture. Following sterilization, the microcarriers were evaluated in 125 mL Corningu spinner cultures at 10 cm2/mL with the Vero African green monkey cell line. To assess cell attachment, distribution, growth and viability, cells were seeded at a density of 2 x 104 cells/cm2 in Dulbecco’s Modified Eagle Medium (DMEM, HyCloneu) +5% fetal bovine serum (FBS, HyClone) + supplements at a stir speed of 40 rpm, in cell culture incubators at 37 ºC, 5% CO2. Cells were harvested at days 2 and 4 via the TrypLEu recombinant dissociation enzyme and counted using a hemacytometer and trypan blue to determine viability. Human Mesenchymal Stem Cell (hMSC) Culture. hMSCs were grown on Star-Plus microcarriers at 10 cm2/mL in HyClone DMEM + 10% FBS and xeno-free medium (Life Technologies) containing 10% human platelet lysate (Cooku Medical) in 2 L stirred-tank reactors. Cells were seeded at 3 x 103 cells/cm2 in the FBS-containing medium, and at 5 x 103 cells/cm2 in the xeno-free medium. Media exchanges (80% of total volume) were performed at multiple timepoints throughout the culture period. Cells were harvested from representative samples at days 2 through 7 and from the entire reactor at day 7 using TrypLE, and counted using a hemacytometer and the trypan blue exclusion method to determine viability. Results Attachment and Distribution. After 2 to 4 hours, both Vero cells (Figure 1) and hMSC attached to the Star-Plus microcarriers in an evenly-distributed fashion across the microcarrier population. No differences were observed between microcarriers sterilized by autoclaving or gamma-irradiation (Figure 1). Microscopic observation indicated that virtually all of the cells were attached to >90% of the microcarriers in a very evenly distributed fashion for both autoclaved and irradiated conditions. Figure 1 Vero Cell Attachment and Distribution. Evenly-distributed Vero cells are visualized by DAPI-stained nuclei on Star-Plus microcarriers. Irradiated 2 Autoclaved Cell Growth. After four days, cell harvest numbers for the Vero cultures reached 1.5 x 105 cells/cm2 with a doubling time of 32.7 hours in the autoclaved condition and 1.6 x 105 cells/cm2 with a doubling time of 31.0 hours in the irradiated condition (Figures 2, 3). Both conditions resulted in greater than 97% viable cells. Figure 2 Vero Cell Growth and Doubling Times. By day 4, healthy and uniformly-distributed cell layers formed on microcarriers in spinner culture under both conditions. Mean values of cells/cm2 +/- SD are shown (n=3). 1.80E+05 1.60E+05 1.40E+05 cells/cm2 1.20E+05 1.00E+05 8.00E+04 Autoclaved 6.00E+04 Irradiated 4.00E+04 2.00E+04 0.00E+00 0 2 4 Days Cells/cm2 Condition Day 0 Day 2 Day 4 Autoclaved Irradiated 2 x 10 2 x 104 4 4.6 x 10 4.8 x 104 4 Doubling Time at Day 4, hrs 1.5 x 10 32.7 1.6 x 10531.0 5 Figure 3 Vero cell growth on microcarriers. Representative phase contrast images of Vero cells on Star-Plus microcarriers 4 days post cell seeding demonstrate that healthy, uniformly distributed cell layers are present in both conditions By day 7, cell harvest numbers for hMSCs reached 7 x 104 cells/cm2 and 8 x 104 cells/cm2 with doubling times of 37 and 42 hours for the FBS-containing and xeno-free media conditions, respectively (Figure 4). A very even distribution of cells across the microcarrier population was observed. Bridging of cells and microcarriers is observed later in the culture period, allowing for cells to grow in the 3-dimensional (3D) space between microcarriers (Figure 5). Both conditions achieved greater than 95% viable cells. The hMSCs retained their differentiation capacity after growth and harvest from the Star-Plus microcarriers. Standard adipogenic and osteogenic differentiation assays were performed. Cells retained their ability to differentiate into adipocytes and osteocytes when grown on Star-Plus microcarriers (Figure 6). www.pall.com/biopharm 3 0 2 3 4 Day 5 6 7 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 9 8 7 6 5 4 3 2 1 0 Xeno-Free Medium 0 3 4 Day 5 6 7 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Cells per mL (x106) FBS-Containing Medium Cells per cm2 (x104) 8 7 6 5 4 3 2 1 0 Cells per mL (x106) Cells per cm2 (x104) Figure 4 Growth and harvest of hMSCs in stirred-tank bioreactors. Arrows indicate when medium exchanges (80% of total volume) occurred. Figure 5 hMSC Attachment and Distribution. Fluorescent images of hMSC DAPI-stained nuclei on Star-Plus microcarriers at day 7 (100x magnification). Figure 6 hMSCs grown in bioreactors retain differentiation capacity. hMSCs grown on Star-Plus microcarriers in serum-containing medium (A) and xeno-free medium (B) were harvested, plated onto flatware and exposed to differentiation medium. A. FBS-Containing Medium Adipocytes Control Diff. Medium 4 Osteocytes B. Xeno-Free Medium Adipocytes Osteocytes Conclusion Star-Plus microcarriers were not only designed to be animal component-free, but also to allow for excellent attachment and growth characteristics from a number of cell line and media combinations. An additional requirement of the design was that the microcarrier be irradiation-tolerant. These attributes enable greater flexibility in how the microcarriers are incorporated into different work flows and processes. Results herein show that attachment, distribution and growth of Vero cells in spinner cultures grown on irradiated Star-Plus microcarriers were similar to that of the autoclaved control. hMSCs grown on Star-Plus microcarriers in both FBS-containing and xeno-free media exhibited uniform attachment and distribution across the microcarrier population, resulting in excellent growth and viability, and importantly retained their differentiation capacity post-harvest from the microcarriers. Pall Life Sciences’ new Star-Plus charged microcarriers are excellent substrates for such applications as cell expansion for the production of cell-based therapies and vaccine manufacturing. Visit us on the Web at www.pall.com/biopharm E-mail us at microcarriers@pall.com Corporate Headquarters Port Washington, NY, USA +1.800.717.7255 toll free (USA) +1.516.484.5400 phone biopharm@pall.com e-mail International Offices Pall Corporation has offices and plants throughout the world in locations such as: Argentina, Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, India, Indonesia, Ireland, Italy, Japan, Korea, Malaysia, Mexico, the Netherlands, New Zealand, Norway, Poland, Puerto Rico, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, Taiwan, Thailand, the United Kingdom, the United States, and Venezuela. Distributors in all major industrial areas of the world. To locate the Pall office or distributor nearest you, visit www.pall.com/contact. European Headquarters Fribourg, Switzerland +41 (0)26 350 53 00 phone LifeSciences.EU@pall.com e-mail The information provided in this literature was reviewed for accuracy at the time of publication. Product data may be subject to change without notice. For current information consult your local Pall distributor or contact Pall directly. Asia-Pacific Headquarters Singapore +65 6389 6500 phone sgcustomerservice@pall.com e-mail © 2015, Pall Corporation. Pall, , and SoloHill are trademarks of Pall Corporation. ® indicates a trademark registered in the USA and TM indicates a common law trademark. Filtration.Separation.Solution. is a service mark of Pall Corporation. uCorning is a trademark of Corning Incorporated, TrypLE is a trademark of Life Technologies Corporation, Cook is a trademark of Cook Incorporated, and HyClone is a trademark of General Electric Company. 11/15, PDF, GN15.6411 www.pall.com/biopharm USD 3086 5