CRYSTAL PPT 0
advertisement

Spring 2007
PHYS 5830: Condensed Matter Physics
Course Code 2536
Instructor: Dr. Tom N. Oder
Physics 5830: Condensed Matter Physics
Course Code 2536
•
•
•
•
•
Dr. Tom N. Oder
WBSH 1016, E-mail: tnoder@ysu.edu,
Phone (330) 941-7111
Website: http://www.ysu.edu/physics/tnoder
Class Website:
www.ysu.edu/physics/tnoder/S07-PHYS2536/index.html
• Office Hours: M, W, F 2:00 pm – 3:00 pm.
• Research: (Wide Band Gap) Semiconductors.
Required Texts:
1. R.F. Pierret: Advanced Semiconductor
Fundamentals, (Second Edition) - Modular
Series on Solid-State Devices, Volume VI,
Addison-Wesley, 1988. By.
2. R. C. Jeager: Introduction to Microelectronics
Fabrication (2nd Edition) - Modular Series on
Solid-State Devices, Volume V, Addison-Wesley.
3. Supplemental reference materials will come
from archival journal papers selected by the
instructor.
Prerequisite: Phys. 3704 Modern Physics. [May be
waived by instructor].
Course Structure: Lecture sessions and
hands-on laboratory activities.
Course Objectives:
1. To develop a background knowledge
of semiconductor theory sufficient to
understand modern semiconductor
devices.
2. To provide students with practical
experience in cutting-edge technology
related to electronic device fabrication
including lithography, thin film deposition
• Adhere to given instructions for safe
handling of processing tools and chemicals.
• Safe handling to avoid equipment damage or
bodily harm.
• Carelessness will lead to removal from class.
• Repair/replacement of a damaged equipment
carelessly handled.
Grading Policy:
Homework/Quizzes 20%.
Midterm Exam (1) 20%.
Week right after Spring break
Laboratory Project 20%.
Final exam 40%.
Homework problems will be
assigned throughout the semester
to reinforce the class material.
Final Grade:
90% - 100% = A
80% - 89% = B
70% - 79% = C
60% - 69% = D
0% - 59%
=F
Cell Phones: Cell phones must be turned
off during class and exam sessions.
Major Areas to be covered
1. Semiconductor Physics
2. Device Physics
3. Processing and Characterization
1 + 2: Mondays, Wednesdays
3: Fridays
Relevant References:
http://ece-www.colorado.edu/~bart/book/book/contents.htm
Semiconductor Physics
•
•
•
•
Crystals: Structure and Growth.
Energy Bands
Carriers in Semiconductors
Phonon Spectra and Optical Properties
of Semiconductors
• Basic Equations for Semiconductor
Device Operation
1. Crystal Structure of Solids
What is “Crystal” to the man on the street?
Significance of Semiconductors
• Computers, palm pilots, laptops, Silicon (Si) MOSFETs,
ICs, CMOS, anything “intelligent”
• Cell phones, pagers Si ICs, GaAs FETs, BJTs
• CD players AlGaAs and InGaP laser diodes, Si
photodiodes
• TV remotes, mobile terminals Light emitting diodes
• Satellite dishes InGaAs MMICs
• Fiber networks InGaAsP laser diodes, pin photodiodes
• Traffic signals, car GaN LEDs (green, blue)
Taillights InGaAsP LEDs (red, amber)
Fundamental Properties of Matter
Matter: - Has mass, occupies space
Mass – measure of inertia - from Newton’s first
law of motion. It is one of the fundamental physical
properties.
States of Matter
1. Solids – Definite volume, definite shape.
2. Liquids – Definite volume, no fixed shape. Flows.
3. Gases – No definite volume, no definite shape.
Takes the volume and shape of its container.
Plasma:
•Regarded as fourth state of matter. No
definite volume, no definite shape. Composed
of electrically charged particles.
•Fully ionized gas at low density with equal
amount of positive and negative charges – net
electrically neutral.
•Affected by electric and magnetic fields.
•Plasma is the main state of matter in
planetary objects such as stars.
Condensate:
•Regarded as fifth state of matter obtained
when atoms/molecules are at very low
temperature and their motion is halted.
•They lose their individual identity and
become a different entity.
•Bose-Einstein condensates – Formed by
bosons.
•Fermionic condensates – By fermions.
Spin
Fermions Half
integral
Bosons
Integral
spin
Occupancy
Examples
Only one per electrons,
state
protons,
neutrons,
quarks,
neutrinos
Many
photons, 4He
allowed
atoms, gluons
Element – one type of atoms
Compound – Two or more different atoms
chemically joined. Constituent atoms (fixed ratios)
can be separated only by chemical means.
Mixture - Two or more different atoms combined.
Constituent atoms (variable ratios) can be separated
by physical means.
Solid-State Physics – branch of physics dealing
with solids.
Now replaced by a more general terminology Condensed Matter Physics. To include fluids which
in many cases share same concepts and analytical
techniques.
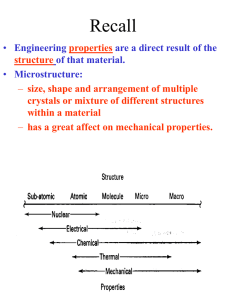
STRUCTURE OF SOLIDS
•Can be classified under several criteria
based on atomic arrangements, electrical
properties, thermal properties, chemical
bonds etc.
•Using electrical criterion: Conductors,
Insulators, Semiconductors
•Using atomic arrangements: Amorphous,
Polycrystalline, Crystalline.
Under what categories could this class be
grouped?
Amorphous Solids
•No regular long range order of arrangement in the
atoms.
•Eg. Polymers, cotton candy, common window glass,
ceramic.
•Can be prepared by rapidly cooling molten material.
•Rapid – minimizes time for atoms to pack into a
more thermodynamically favorable crystalline state.
•Two sub-states of amorphous solids: Rubbery and
Glassy states. Glass transition temperature Tg =
temperature above which the solid transforms from
glassy to rubbery state, becoming more viscous.
Amorphous Solids
Continuous random network structure of atoms
in an amorphous solid
Polycrystalline Solids
•Atomic order present in sections (grains)
of the solid.
•Different order of arrangement from
grain to grain. Grain sizes = hundreds of
m.
•An aggregate of a large number of small
crystals or grains in which the structure is
regular, but the crystals or grains are
arranged in a random fashion.
Polycrystalline Solids
Crystalline Solids
Atoms arranged in a 3-D long range order.
“Single crystals” emphasizes one type of
crystal order that exists as opposed to
polycrystals.
Single- Vs Poly- Crystal
• Properties of single crystalline materials
vary with direction, ie anisotropic.
•Properties of polycrystalline materials
may or may not vary with direction.
If the polycrystal grains are randomly
oriented, properties will not vary with
direction ie isotropic.
•If the polycrystal grains are textured,
properties will vary with direction ie
anisotropic
Single- Vs Poly- Crystal
Single- Vs Poly- Crystal
200 m
-Properties may/may not
vary with direction.
-If grains are randomly
oriented: isotropic.
(Epoly iron = 210 GPa)
-If grains are textured,
anisotropic.
Solid state devices employ semiconductor
materials in all of the above forms.
Examples:
Amorphous silicon (a-Si) used to make thin film
transistors (TFTs) used as switching elements in
LCDs.
Ploycrystalline Si – Gate materials in
MOSFETS.
Active regions of most solid state devices are
made of crystalline semiconductors.
Hard Sphere Model of Crystals
• Assumes atoms are hard spheres with
well defined diameters that touch.
•Atoms are arranged on periodic array –
or lattice
•Repetitive pattern – unit cell defined by
lattice parameters comprising lengths of
the 3 sides (a, b, c) and angles between the
sides (, , ).
Lattice Parameters
c
a
b
Atoms in a Crystal
The Unit Cell Concept
•The simplest repeating unit in a crystal is called a
unit cell.
•Opposite faces of a unit cell are parallel.
•The edge of the unit cell connects equivalent
points.
•Not unique. There can be several unit cells of a
crystal.
•The smallest possible unit cell is called primitive
unit cell of a particular crystal structure.
•A primitive unit cell whose symmetry matches
the lattice symmetry is called Wigner-Seitz cell.
• Each unit cell is defined in terms of lattice points.
•Lattice point not necessarily at an atomic site.
• For each crystal structure, a conventional unit cell,
is chosen to make the lattice as symmetric as
possible. However, the conventional unit cell is not
always the primitive unit cell.
•A crystal's structure and symmetry play a role
in determining many of its properties, such as
cleavage (tendency to split along certain planes
with smooth surfaces), electronic band structure
and optical properties.
Unit cell
b
a
•Unit cell: Simplest portion of the structure which is
repeated and shows its full symmetry.
•Basis vectors a and b defines relationship between a
unit cell and (Bravais) lattice points of a crystal.
•Equivalent points of the lattice is defined by translation
vector.
r = ha + kb where h and k are integers. This constructs
the entire lattice.
•By repeated duplication, a unit cell should
reproduce the whole crystal.
•A Bravias lattice (unit cells) - a set of points
constructed by translating a single point in
discrete steps by a set of basis vectors.
•In 3-D, there are 14 unique Bravais lattices.
All crystalline materials fit in one of these
arrangements.
In 3-D, the translation vector is
r = ha + kb + lc
Crystal System
•The crystal system: Set of rotation and
reflection symmetries which leave a lattice
point fixed.
•There are seven unique crystal systems: the
cubic (isometric), hexagonal, tetragonal,
rhombohedral (trigonal), orthorhombic,
monoclinic and triclinic.
Bravais Lattice and Crystal System
Crystal structure: contains atoms at every lattice
point.
•The symmetry of the crystal can be more
complicated than the symmetry of the lattice.
•Bravais lattice points do not necessarily
correspond to real atomic sites in a crystal. A
Bravais lattice point may be used to represent a
group of many atoms of a real crystal. This means
more ways of arranging atoms in a crystal lattice.
1. Cubic (Isometric) System
3 Bravais lattices
Symmetry elements: Four 3-fold rotation axes along
cube diagonals
c
a=b=c
b
a
o
= = = 90
By convention, the edge of a unit cell always
connects equivalent points. Each of the eight
corners of the unit cell therefore must contain an
identical particle.
(1-a): Simple Cubic Structure (SC)
• Rare due to poor packing (only Po has this structure)
• Close-packed directions are cube edges.
Coordination # = 6
(# nearest neighbors)
1 atom/unit cell
Coordination Number = Number of
nearest neighbors
One atom per unit cell
1/8 x 8 = 1
Atomic Packing Factor
• APF for a simple cubic structure = 0.52
a
R=0.5a
Adapted from Fig. 3.19,
Callister 6e.
(1-b): Face Centered Cubic Structure (FCC)
• Exhibited by Al, Cu, Au, Ag, Ni, Pt
• Close packed directions are face diagonals.
• Coordination number = 12
• 4 atoms/unit cell
All atoms are identical
Adapted from Fig. 3.1(a),
Callister 6e.
6 x (1/2 face) + 8 x 1/8 (corner) = 4 atoms/unit cell
FCC
Coordination number = 12
3 mutually perpendicular planes.
4 nearest neighbors on each of the three planes.
How is a and R related for an FCC?
[a= unit cell dimension, R = atomic radius].
All atoms are identical
(1-c): Body Centered Cubic Structure (BCC)
• Exhibited by Cr, Fe, Mo, Ta, W
• Close packed directions are cube diagonals.
• Coordination number = 8
All atoms are identical
2 atoms/unit cell
How is a and R related for an BCC?
[a= unit cell dimension, R = atomic radius].
All atoms are identical
2 atoms/unit cell
Which one has most packing ?
Which one has most packing ?
For that reason, FCC is also referred to
as cubic closed packed (CCP)
2. Hexagonal System
Only one Bravais lattice
Symmetry element: One 6-fold rotation axis
a=bc
= 120o
= = 90o
Hexagonal Closed Packed Structure (HCP)
• Exhibited by ….
• ABAB... Stacking Sequence
• Coordination # = 12
• APF = 0.74
3D Projection
A sites
B sites
A sites
Adapted from Fig. 3.3,
Callister 6e.
2D Projection
3. Tetragonal System
Two Bravais lattices
Symmetry element: One 4-fold rotation axis
a=bc
= = = 90o
4. Trigonal (Rhombohedral) System
One Bravais lattice
Symmetry element: One 3-fold rotation axis
a=bc
= 120o
= = 90o
5. Orthorhombic System
Four Bravais lattices
Symmetry element: Three mutually perpendicular 2fold rotation axes
abc
= = = 90o
6. Monoclinic System
Two Bravais lattices
Symmetry element: One 2-fold rotation axis
abc
= = 90o, 90o
7. Triclinic System
One Bravais lattice
Symmetry element: None
abc
90o
•The crystal system: Set of
symmetries which leave a lattice point
fixed. There are seven unique crystal
systems.
• Some symmetries are identified by
special name such as zincblende,
wurtzite, zinc sulfide etc.
Layer Stacking Sequence
A sites
HCP
B sites
A sites
= ABAB…
= ABCABC..
FCC
FCC: Coordination number
FCC
Coordination number = 12
3 mutually perpendicular planes.
4 nearest neighbors on each of the three planes.
Diamond Lattice Structure
•Exhibited by Carbon (C), Silicon (Si) and
Germanium (Ge).
•Consists of two interpenetrating FCC lattices,
displaced along the body diagonal of the cubic
cell by 1/4 the length of the diagonal.
• Also regarded as an FCC lattice with two
atoms per lattice site: one centered on the
lattice site, and the other at a distance of a/4
along all axes, ie an FCC lattice with the twopoint basis.
Diamond Lattice Structure
a = lattice constant
Diamond Lattice Structure
Two merged FCC cells offset by a/4 in x, y and z.
Basic FCC Cell
Merged FCC Cells
Omit atoms
outside Cell
Bonding of Atoms
8 atoms at each corner, 6 atoms on each
face, 4 atoms entirely inside the cell
Zinc Blende
•Similar to the diamond cubic structure
except that the two atoms at each lattice
site are different.
• Exhibited by many semiconductors
including ZnS, GaAs, ZnTe and CdTe.
•GaN and SiC can also crystallize in this
structure.
Zinc Blende
Each Zn bonded to 4 Sulfur
- tetrahedral
Equivalent if Zn and S are
reversed
Bonding often highly covalent
Zinc sulfide crystallizes in two different forms:
Wurtzite and Zinc Blende.
GaAs
Red = Ga-atoms, Blue = As-atoms
•Equal numbers of Ga and As ions distributed on
a diamond lattice.
• Each atom has 4 of the opposite kind as nearest
neighbors.
Wurtzite (Hexagonal) Structure
•This is the hexagonal analog of the zinc-blende
lattice.
• Can be considered as two interpenetrating
close-packed lattices with half of the tetrahedral
sites occupied by another kind of atoms.
• Four equidistant nearest neighbors, similar to a
zinc-blende structure.
•Certain compound semiconductors (ZnS, CdS,
SiC) can crystallize in both zinc-blende (cubic)
and wurtzite (hexagonal) structure.
WURTZITE
A sites
B sites
A sites
Wurtzite Gallium Nitride (GaN)
Miller Index
Step 1 : Identify the intercepts on the x- , y- and z- axes.
Step 2 : Specify the intercepts in fractional co-ordinates
Step 3 : Take the reciprocals of the fractional intercepts
(i) in some instances the Miller indices are best multiplied or divided
through by a common number in order to simplify them by, for
example, removing a common factor. This operation of multiplication
simply generates a parallel plane which is at a different distance from
the origin of the particular unit cell being considered.
e.g. (200) is transformed to (100) by dividing through by 2 .
(ii) if any of the intercepts are at negative values on the axes then the
negative sign will carry through into the Miller indices; in such cases
the negative sign is actually denoted by overstriking the relevant
number.
e.g. (00 -1) is instead denoted by 00 1
Miller Index For Cubic Structures
•Miller index is used to describe directions and
planes in a crystal.
•Directions - written as [u v w] where u, v, w.
• Integers u, v, w represent coordinates of the
vector in real space.
•A family of directions which are equivalent due
to symmetry operations is written as <u v w>
•Planes: Written as (h k l).
•Integers h, k, and l represent the intercept of the
plane with x-, y-, and z- axes, respectively.
• Equivalent planes represented by {h k l}.
Miller Indices: Directions
z
y
x
[1] Draw a vector and take components
[2] Reduce to simplest integers
[3] Enclose the number in square brackets
x
0
0
y
2a
1
[0 1 1]
z
2a
1
Negative Directions
z
y
x
[1] Draw a vector and take components
[2] Reduce to simplest integers
[3] Enclose the number in square brackets
x
0
0
y
-a
-1
0 1 2
z
2a
2
Miller Indices: Equivalent Directions
Equivalent directions due to crystal symmetry:
z
1:
2:
3:
[100]
[010]
[001]
3
y
x
2
1
Notation <100> used to denote all directions equivalent to [100]
Directions
Directions
_
[1 0 1]
_
[1 2 1]
The intercepts of a crystal plane with the axis defined by a set of
unit vectors are at 2a, -3b and 4c. Find the corresponding Miller
indices of this and all other crystal planes parallel to this plane.
The Miller indices are obtained in the following three steps:
1. Identify the intersections with the axis, namely 2, -3 and 4.
2. Calculate the inverse of each of those intercepts, resulting in
1/2, -1/3 and 1/4.
3. Find the smallest integers proportional to the inverse of the
intercepts. Multiplying each fraction with the product of
each of the intercepts (24 = 2 x 3 x 4) does result in integers,
but not always the smallest integers.
4. These are obtained in this case by multiplying each fraction
by 12.
5. Resulting Miller indices is 6 4 3
6. Negative index indicated by a bar on top.
z
Miller Indices of Planes
z=
y
x=a
y=
x
x
y
z
∞
0
[2] Invert the intercept values
1/a
∞
1/∞
[3] Convert to the smallest integers
1
0
[1] Determine intercept of plane with each axis a
[4] Enclose the number in round brackets
(1 0 0)
1/∞
Miller Indices of Planes
z
y
x
x
[1] Determine intercept of plane with each axis 2a
[2] Invert the intercept values
1/2a
[3] Convert to the smallest integers
1
[4] Enclose the number in round brackets
y
z
2a
1/2a
2a
1/2a
1
1
(1 1 1)
z
Planes with Negative Indices
y
x
x
[1] Determine intercept of plane with each axis a
[2] Invert the intercept values
1/a
[3] Convert to the smallest integers
1
[4] Enclose the number in round brackets
y
z
-a
-1/a
a
1/a
-1
1 1 1
-1
z
Equivalent Planes
(100)
plane
(001) plane
(010)
plane
y
x
• Planes (100), (010), (001), (100), (010), (001)
are equivalent planes. Denoted by {1 0 0}.
• Atomic density and arrangement as well as
electrical, optical, physical properties are also
equivalent.
(0 1 1)
The (110) surface
Assignment
Intercepts : a , a ,
Fractional intercepts : 1 , 1 ,
Miller Indices : (110)
The (100), (110) and (111) surfaces considered above are
the so-called low index surfaces of a cubic crystal system
(the "low" refers to the Miller indices being small numbers 0 or 1 in this case).
Crystallographic Planes
Miller Indices (hkl)
reciprocals
Crystallographic Planes
_
(1 1 1)
in the cubic system the (hkl) plane and the vector
[hkl], defined in the normal fashion with respect
to the origin, are normal to one another but this
characteristic is unique to the cubic crystal
system and does not apply to crystal systems of
lower symmetry
Family
of
Planes
All planes that are identical are included in 1 family
denoted by { hkl}
Example {111}
(111) and (111) have equivalent
atoms
The (111) surface
Assignment
Intercepts : a , a , a
Fractional intercepts : 1 , 1 , 1
Miller Indices : (111)
The (210) surface
Assignment
Intercepts : ½ a , a ,
Fractional intercepts : ½ , 1 ,
Miller Indices : (210)
Symmetry-equivalent surfaces
the three highlighted surfaces
are related by the symmetry
elements of the cubic crystal they are entirely equivalent.
In fact there are a total of 6 faces related by the
symmetry elements and equivalent to the (100) surface any surface belonging to this set of symmetry related
surfaces may be denoted by the more general notation
{100} where the Miller indices of one of the surfaces is
instead enclosed in curly-brackets.
in the hcp crystal system there are four principal
axes; this leads to four Miller Indices e.g. you may
see articles referring to an hcp (0001) surface. It is
worth noting, however, that the intercepts on the
first three axes are necessarily related and not
completely independent; consequently the values
of the first three Miller indices are also linked by a
simple mathematical relationship
In case of a cubic structure, the Miller index of a plane, in
parentheses such as (100), are also the coordinates of the direction
of a plane normal. It stands for a vector perpendicular to the family
of planes, with a length of d-1, where d is the inter-plane spacing.
Due to the symmetries of cubic crystals, it is possible to change the
place and sign of the integers and have equivalent directions and
planes:
•Coordinates in angle brackets or chevrons such as <100>
denote a family of directions which are equivalent due to
symmetry operations. If it refers to a cubic system, this
example could mean [100], [010], [001] or the negative of any
of those directions.
•Coordinates in curly brackets or braces such as {100} denote
a family of plane normals which are equivalent due to
symmetry operations, much the way angle brackets denote a
family of directions.
QUESTIONS
1.
With hexagonal and rhombohedral crystal systems, it is possible
to use the Bravais-Miller index which has 4 numbers (h k i l)
i = -h-k
where h, k and l are identical to the Miller index.
The (100) plane has a 3-fold symmetry, it remains unchanged by a
rotation of 1/3 (2π/3 rad, 30°). The [100], [010] and
the
directions are similar. If S is the intercept of the plane
with the
axis, then
i = 1/S
i is redundant and not necessary.
in the hcp crystal system there are four principal axes; this leads to
four Miller Indices e.g. you may see articles referring to an hcp
(0001) surface. It is worth noting, however, that the intercepts on
the first three axes are necessarily related and not completely
independent; consequently the values of the first three Miller
indices are also linked by a simple mathematical relationship.
In the cubic crystal system, a plane and the direction normal to
it have the same indices.
[101] direction is normal to the plane (101)
a
Distance (d) separating adjacent planes (hkl) d
of a cubic crystal of lattice constant (a) is:
Angle () between
directions [h1 k1 l1]
and [h2 k2 l2] of a
cubic crystal is:
cos( )
h2 k 2 l 2
h1h2 k1k2 l1l2
(h1 k1 l1 )( h2 k2 l2 )
2
2
2
2
2
2
Miller-Bravais Indices
• For hcp crystal structure
• Planes (h k i l), directions [h k i l]
• Sum of the first three indices h + k + i = 0
To determine the Miller-Bravais indices
of the crystallographic direction
indicated on the a1-a2-a3 plane of an
h.c.p. unit cell (ie. c = 0), the vector
must be projected onto each of the
three axes to find the corresponding
component, such that:
CRYSTAL GROWTH
Seed
Ingot
Buole
Miller Indices – Silicon Wafers
(100)
plane
(surface)
Flat
edg
e
[011]
direction
Flats are cut out of wafers to indicate crystal orientation of
wafer surface
Essential to know surface orientation of Si wafer if you
want to fabricate circuits on it
ATOMIC PACKING FACTOR:
BCC
• APF for a body-centered
cubic structure = 0.68
Close-packed direction - diagonal
a=
R
Adapted from
Fig. 3.2,
Callister 6e.
a
4R
3
Calculate the maximum fraction of the volume in a simple cubic
crystal occupied by the atoms. Assume that the atoms are closely
packed and that they can be treated as hard spheres. This fraction
is also called the packing density.
The atoms in a simple cubic crystal are located at the corners of the
units cell, a cube with side a. Adjacent atoms touch each other so
that the radius of each atom equals a/2. There are eight atoms
occupying the corners of the cube, but only one eighth of each is
within the unit cell so that the number of atoms equals one per unit
cell. The packing density is then obtained from:
or about half the volume of the unit cell is occupied by the atoms.
The packing density of four cubic crystals is listed in the table
below.
ATOMIC PACKING FACTOR:
FCCcubic structure = 0.74
• APF for a face-centered
Close-packed directions (diagonal):
length = 4R
a = 2R 2
Unit cell contains
4 atoms/unit cell
Adapted from
Fig. 3.1(a),
Callister 6e.
atoms
4
unit cell
APF =
4 p 3
R
3
16
R3
2
volume
atom
volume
unit cell