Atomic Arrangement Reading Assignment 39
advertisement

Recall
• Engineering properties are a direct result of the
structure of that material.
• Microstructure:
– size, shape and arrangement of multiple
crystals or mixture of different structures
within a material
– has a great affect on mechanical properties.
Levels of Atomic
Arrangement
Definitions
Amorphous
Crystalline
• No long range
order, short range
atomic order (1 -2
atomic diameters)
• Long range order of
atoms
Unit Cell
• Basic building block of Crystal
Structure
• Repeated through space
• Like a Lego piece in a Lego
building
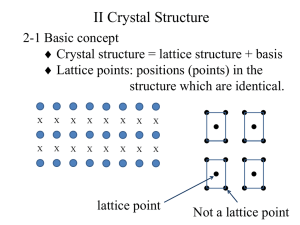
Describing the Crystal
Lattice
• Lattice Points
• Lattice Parameters
– a, b, c, describe length of
sides
– a. b. g, describe angles
between sides
Bravais Lattices
Common Crystal Structures of Metals
Body Centered Cubic
Example - Steel
Common Crystal Structures of Metals
Face Centered Cubic
Example – aluminum and steel
Common Crystal Structures of Metals
Hexagonal Close Packed
Example – titanium, some ceramics
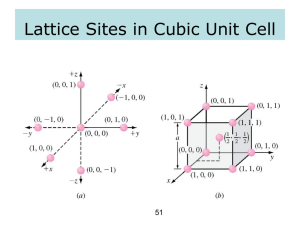
Coordinates of Points
Miller Indices - Directions
1 – Identify the location (coordinates of points)
for the arrow head and tail.
2- Subtract the head from the tail
3- Clear any fractions
4- Put a line over any negative values
5- Enclose in “[ ]”
Group work
• Use Miller Indices to identify
the following directions
(001)
(011)
(101)
(010)
(100)
(110)
(001)
(011)
(101)
(010)
(100)
(110)
• 1 0 ½ - 0 ½ 1 =[1 -1/2 -1/2]
=[2-1-1] (place line over neg
values)
• 011 – 100 = [-111]
• ½ 00 – 010 = [1/2 -1 0]= [1-20]
• How did you do?
Directions of Form
• Generic directions – ex diagonal
of the face
Directions of Form
• Generic directions can be noted
using < > instead of [ ];
Close packed direction
• Direction on a unit cell in a
crystal where all of the atoms
are touching!
• For FCC this is the <101>
• For BCC this is <111>
Miller Indices - Planes
• Determine the intercepts of the plane
on the crystallographic axes; If the
plane intercepts the axis at the origin,
then the origin must be moved to
another location, If the plane does not
intersect a particular axes then the
intercept is considered to be infinity.
• Take the reciprocal of the intercepts.
• Clear any fractions;
• Enclose values of h, k and l in
parenthesis, indicate negative values
by placing a bar over that value.
Group Work
• Determine the Miller Indices for
the following plane
1/3
1/3
• Example 1
• X = infinity
• Y = 1/3
• Z = infinity
– Reciprocal
• X=0
• Y=3
• Z=0
– No fractions to clear, no negative
values
– (030) planes = parenthesis
• Example 2 (move origin to 001)
• X=1
• Y =infinity
• Z = - 1/3
– Reciprocal
• X=1
• Y=0
• Z = -3
– No fractions to clear, negative
values , put line over number
– (10-3) planes = parenthesis
• Example 3 (move origin to 010)
• X=1
• Y = -1
• Z=1
– Reciprocal
• X=1
• Y = -1
• Z=1
– No fractions to clear, negative
values , put line over number
– (1 -1 1) planes = parenthesis
Planes of Form
Group Work
• Determine the Close Packed
Plane for an FCC unit cell
(draw it and use Miller indices
to define)
• Determine the close packed
plane for a BCC (hint this is a
trick question, why?)
Looks like this….
This
Close packed plane is of the form {111} see
previous example
Close Packed Planes
Who Cares?
• The mechanism for plastic
deformation most often occurs
on close packed planes in close
packed directions and that is
why we care!!!
• More close packed planes and
directions => easier to plastic
deform…think of Aluminum
and Steel…does this make
sense?
Atoms per Unit Cell
• Atoms are shared between unit
cells
• How many atoms/unit cell does
a BCC crystal structure have?
• How many atoms/unit cell does
an FCC crystal structure have?
Unit Cell 1
Unit Cell 3
Atom 1
Unit Cell 2
Unit Cell 4
Repeat Distance – Distance
between two atoms
Repeat
distance =
lattice
parameter
Repeat
distance = ½
diagonal of
face
Describing the Packing
Efficiency of aCrystal
Lattice
• Coordination Number – number of
nearest neighbors – speaks to how
efficiently packed a unit cell is
• Packing Fraction
– Linear
– Planar
• Density
– Linear
– Planar
– Material
Miller-Bravais Indices
Development of a Grain
Structure
• Crystals or grains: small continuous
volumes of solid;
• Nucleus
• Basic lattice is repeated through space;
• Grain boundaries
• Nucleation and growth
• Number and size of grains
– fast nucleation rate => small grains
– fast growth rate => large grains
– grain structure affects mechanical properties