Tipler Chapters 37 & 38 Name: #1 Ionic Bonding of Rubidium
advertisement

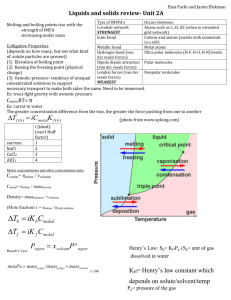
Tipler Chapters 37 & 38 Name: ________________________ #1 Ionic Bonding of Rubidium Fluoride The dissociation energy of rubidium fluoride (RbF) is 5.12 eV and the equilibrium separation of Rb F is 0.227 nm. The electron affinity of a fluorine atom is –3.40 eV and the ionization energy of rubidium is 4.18 eV. Determine the core-repulsion energy of RbF. #2 Lenard-Jones Potential Function The potential energy between two atoms in a molecule separated by a distance r is often described by the Lenard-Jones potential function: 𝑎 12 𝑟 𝑈 = 𝑈0 [( ) 𝑎 6 − ( ) ], 𝑟 where U0 and a are empirical constants. Find an expression for the equilibrium separation r0 in terms of a. (Hint: Find the separation distance where the function U(r) is a minimum.) #3 Packing Fraction In a crystalline solid, the atoms that make up the material are arranged in a periodic fashion. A given orderly arrangement of atoms is called a lattice. The simplest three-dimensional lattice is the simple cubic (SC) arrangement. In this arrangement, the atoms are located at the corners of a cube. Two other cubic structures include the body-centered cubic (BCC) in which there is an additional atom at the geometric center of the cube, and the face-centered cubic (FCC) which, in addition to the atoms at the eight corners of the cube, there are atoms centered on each of the six faces of the cube. (a) Consider the simple cubic lattice, face-centered cubic lattice and a body-centered cubic lattice shown above. How many whole atoms are actually found inside each lattice cube (also known as the unit cell)? For the following calculations, assume that the atoms in a particular crystalline material are identical and can be treated as perfectly rigid spheres that pack as tightly as geometrically possible. The packing fraction of a crystal lattice is defined as the ratio of the volume taken up by the atoms within a unit cell to the volume of the unit cell. (b) Again consider the three lattices shown above. Calculate the packing fraction for each of the three lattice structures assuming that that each cube has an edge length of a? (c) Which cubic structure is has the most efficient packing? #4 Conductor or Insulator Consider a hypothetical element that forms a solid with bands as shown in the figure to the left. Suppose that the isolated atom has the electron configuration 1s22s2. (a) At an equilibrium separation of ra, is the solid a conductor or insulator? What if the equilibrium distance is rb? Defend your answer in each case. (b) Repeat Part (a) for the same bands, only this time for atoms having the electron configuration 1s22s22p1. (c) Repeat Part (a) for the same bands, only this time for atoms having the electron configuration 1s22s22p6.