Acid Base Balance of Human body
advertisement
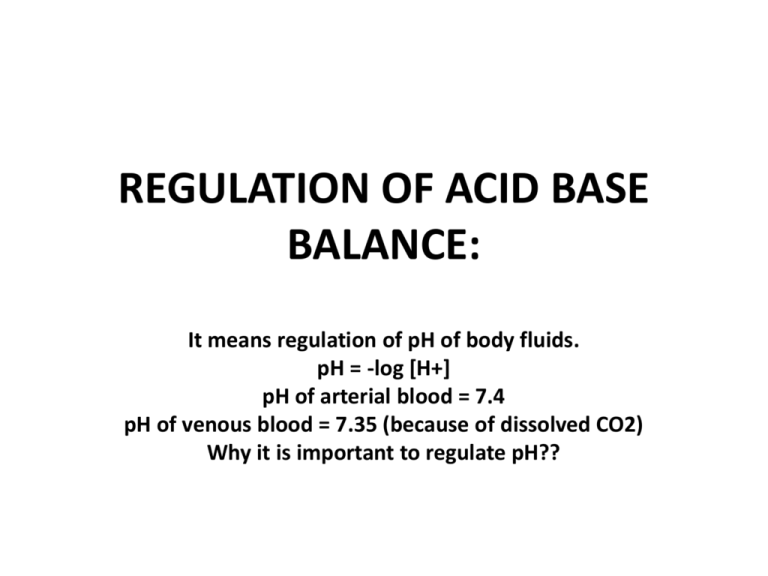
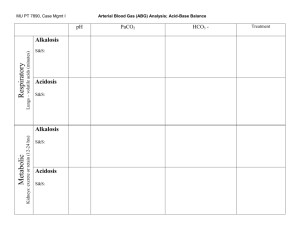
REGULATION OF ACID BASE BALANCE: It means regulation of pH of body fluids. pH = -log [H+] pH of arterial blood = 7.4 pH of venous blood = 7.35 (because of dissolved CO2) Why it is important to regulate pH?? • It is important to regulate pH because enzymes in body need optimal pH & when pH changes, there is marked effect on activity of enzymes. • When pH is >7.45, it is ALKALOSIS • When pH is <7.35, it is ACIDOSIS • In which range of pH, person can survive? • Survival range: (very narrow range) • pH of body fluids depend upon BUFFERS in body fluids. • What is a BUFFER SYSTEM? • Buffer system is a solution which minimize or resist change in pH (IT CANNOT PREVENT THE CHANGE!!!) • A buffer system consists of a weak acid & its salt (mostly) or a weak alkali & its salt, e . g, HCO3- buffer: • salt is NaHCO3 & weak acid is H2CO3 • It may be KHCO3 salt…..but in plasma & ECF main cation is Na+ • Another e . g, is PO4 --- buffer: • salt is Na2HPO4 & acid is NaH2PO4 • ACID: Which can donate H+ • BASE: Which can accept H+ BUFFERS: • 1) BLOOD (Plasma & RBCs) • 2) IN ISF • 3) IN ICF BLOOD BUFFERS: • 1) HCO3 BUFFER • 2) PO4 BUFFER • 3) PROTEIN BUFFER • 4) Hb BUFFER IN RBCs HCO3 BUFFER IN BLOOD: • It consist of HCO3 (salt) & acid (H2CO3 which is dissolved CO2). • HCO3 means NaHCO3 because Na+ is main cation in plasma. • Ratio = salt/acid = NaHCO3/H2CO3 = 20/1 pka of this buffer system (HCO3) = 6.1 6.1 + log20 = 6.1 + 1.3 = 7.4 pka = -log of dissociation constant of acid HENDERSON HASSEL BALCH EQUATION: • pH = pka + log [salt / acid] • pka is –log of dissociation constant of acid • HCO3 buffer system: for HCO3-, pka = 6.1 so 6.1 + log salt or HCO3 Acid = 6.1 + log 24mEq or mM/L 1.2mEq or mM/L = 6.1 + log 20 = 6.1 + 1.3 = 7.4 When ratio between salt & acid is 20, pH will be 7.4. PO4 BUFFER IN BLOOD: • SALT= Na2HPO4 • ACID= NaH2PO4 • Ratio=salt/acid = 4/1 • Pka of H3PO4 = 6.8 PROTEIN BUFFER IN BLOOD: • SALT=Na-Proteinate • ACID=Acid Protein or H-Protein Hb BUFFER IN RBCs • Cation in RBCs = K+ • For Hb buffer, salt = K-Hemoglobinate • Acid = Acid Hemoglobin=HHb • Hb is very important buffer in blood BUFFERS IN ISF: • HCO3 • PO4 • Weak protein buffer BUFFER IN ICF: • Main buffer in ICF is protein buffer. • Buffering power of a buffer depends on 2 factors: 1) conc. of buffer (quantitative) 2) pka (qualitative) If conc. is greater, stronger will be the buffer. If pka of buffer is near to pH of blood, stronger will be that buffer. If we compare HCO3 & PO4 buffer, quantitatively powerful is HCO3 buffer. Its conc. is 10x more than PO4 buffer. Qualitatively, PO4 buffer is more powerful, as 6.8 is closer to 7.4 than 6.1. Sources of H+ or acid in the body: 1) OXIDATION OF CARBON CONTAINING COMPOUNDS: • Gives rise to CO2 (Volatile acid). • During exercise production of CO2 increases very much. 2) FORMATION OF NON-VOLATILE OR ORGANIC ACIDS DURING METABOLISM OF CHO, FATS & PROT. - Most of these acids are further oxidized to form CO2 & H2O, but their level increase in the blood when there is increased rate of metabolism. - In hypoxia increased production of these acids. (to provide energy, rapid metabolism) - Certain drugs & disorders can increase their production. - These acids are PYRUVIC ACID, LACTIC ACID, ACETOACETIC ACID & BETA HYDROXY BUTYRIC ACID. 3) FORMATION OF H2SO4: • When S containing compounds (like Cysteine & Methionine) are oxidized, H2SO4 is produced. 4) FORMATION OF H3PO4: • When phospho-esters, phosphatides. Phospho-proteins & nucleo-proteins are hydrolyzed in the body. 4) Small amount of some acids are INGESTED BY MOUTH: • Like NH4Cl in cough syrup (noshadir) mild acidosis. BUFFERING MECHANISMS IN THE BODY: (2 TYPES) • 1) PHYSICO-CHEMICAL BUFFERING • 2) PHYSIOLOGICAL BUFFERING PHYSICO-CHEMICAL BUFFERING: • Most immediate buffering. (when an acid or alkali is added to body fluids, it is the 1st line of defense against disturbance of acid base balance. A) PHYSICO-CHEMICAL BUFFERING OF CO2/VOLATILE ACID: CO2 is transported as HCO3 & in free form. From tissues, CO2 RBCs. In RBCs, CO2 + H2O H2CO3 H2CO3 (unstable) H + HCO3 H ion + Hb HHb (buffered by Hb to form acid-Hb). • At tissue level, deoxy Hb is available. DeoxyHb can bind much more H than oxy-Hb (already acidic). • HCO3 diffuses out into plasma & from plasma, Cl diffuse in to maintain electrical balance. This is HCO3-Cl SHIFT OR HAMBERGER’S SHIFT. • Some CO2 combine with amino group of Hb to form CARBAMINO-Hb. • Some CO2 binds with amino group of plasma proteins to form CARBAMINO-PROTEINS. Cl Hb H HCO3 carbamino-Hb H2CO3 CO2 + H20 (CA) carbamino-proteins CO2 RBC B) PHYSICO-CHEMICAL BUFFERING OF ORGANIC/NON-VOLATILE ACIDS: • Carried out by various chemical buffers in body fluids like HCO3 & PO4 buffers. • e.g in body there is production of H3PO4, so NaHCO3 will buffer it & we get Na2HPO4 + H2CO3. H3PO4 + NaHCO3 Na2HPO4 + H2CO3. (strong acid) (salt) (weak acid) CONCLUSION: In case of volatile acids buffering: Hb & plasma proteins play a role. Incase of organic/non-volatile acid buffering: NaHCO3 is utilized, which must be replenished & body must get rid of acid anion/salt & the weak acid; H2CO3. ROLE OF PHYSIOLOGICAL BUFFERING: • It is actually to deal with end product of physico-chemical buffering. • In physiological buffering, there is role of respiratory system & renal system. ROLE OF RESPIRATORY SYSTEM: • It removes CO2 from the body. Also removes dissolved CO2 (i-e H2CO3). • When RBC goes to lung capillaries, blood becomes oxygenated. • O2 enters the RBC. O2 binds with Hb to form oxy-Hb (strong acid), which cannot hold H). • There is reverse HCO3-Cl shift (lung level). • From plasma HCO3 move into RBC & Cl move in reverse direction. • CO2 from Carbamino Hb also comes out. • In RBC there is reverse reaction. H + HCO3 H2CO3 CO2 + H2O. CO2 alveoli expired out. • As a result of physiological buffering, Hb & plasma proteins are again available to buffer CO2 or H. (RECYCLING) • CO2 is very strong stimulant of respiratory centre. • Buffering by respiratory system takes minutes to hours. • Buffering power of resp. system is 1 to twice more powerful, as compared to buffering by chemical buffers in body fluids (HCO3, PO4 buffers etc). ROLE OF RENAL SYSTEM: • Kidneys regenerate HCO3. • There is reabsorption of Na & Cl ions when required. • Acid anions or salts are excreted in urine. • There is secretion of H & Ammonia by the kidney. 3) METABOLIC ACIDOSIS: • Due to increase H ion production in body, conc of HCO3 decreases. So salt/acid = HCO3/PCO2 = decreased ratio decreased pH, because of less HCO3 in arterial blood. So pH decreases to produce metabolic acidosis. CAUSES OF METAB ACIDOSIS: • 1) FAILURE TO EXCRETE normally produced metab acids in urine. In chronic renal failure, kidneys cannot excrete normally produced metab acids. • 2) INCREASED PRODUCTION OF METAB/ORGANIC ACIDS: e.g uncontrolled DM, Severe hypoxia (lactic acidosis). • 3) LOSS OF ALKALINE FLUID FROM BODY:e.g Severe diarrhoea, intestinal fistula & vomiting of intestinal contents. • 4) HYPERKALEMIA: In hyperkalemia, body tends to excrete K ion, instead of H ion. So H ion is conserved acidosis. (IN HYPERKALEMIA THERE IS ACIDOSIS). • 5) CARBONIC ANHYDRASE INHIBITORS: e.g Acetazolamide. H ions are not secreted & no reabs of HCO3 Metabolic acidosis. COMPENSATION OF MET ACIDOSIS: • 1) Various buffers in body fluids, buffer the excess of H ion,e.g, HCO3 buffer, PO4 buffer & protein buffer. • 2) Resp system: Because of increased H ion conc. hyperventillation loss of CO2 less PCO2 Ratio will increase back to normal & pH will increase back to normal. • *In compensated cases of metab acidosis, there is some resp alkalosis to decrease PCO2 because of hyperventillation. • 3) Renal compensation: Kidney secretes H ion in large amount. There is increased NH3 secretion. There is increased HCO3 reabsorption or regeneration. When there is more HCO3 reabsorption, Cl is lost in urine. 4) METABOLIC ALKALOSIS: • There is more HCO3 conc. in arterial blood, so ratio between salt & acid increases, so pH will increase to produce metabolic alkalosis. • CAUSES: • 1) Ingestion of large amount of alkali ,e.g, in gastritis & peptic ulcer as a treatment. • 2) Vomiting of gastric contents, due to loss of acids from stomach in large amounts. • 3) Increase of Aldosterone: Increased Na reabs which is coupled with counter transport of K & also H, so when there is increased aldosterone hypokalemia & alkalosis. EFFECTS OF ACIDOSIS & ALKALOSIS ON BODY: • EFFECT OF ACIDOSIS: When pH decreases CNS is depressed patient becomes disoriented, drowsy & comatosed in severe cases, e.g, diabetic coma of ketoacidosis Kussmal breathing (rapid & deep breathing) with ketotic breath. • EFFECT OF ALKALOSIS: When ionic calcium decreases hypocalcemia tetany (hyperexcitability of nerves) carpopedal & laryngeal spasm, convulsions, paresthesias due to involvement of sensory nerves. CLINICAL EVALUATION OF ACID BASE BALANCE: -1) MEASUREMENT OF ARTERIAL pH: (1st parameter) = 7.4 -2) MEASUREMENT OF ARTERIAL PCO2:= 40mmHg -3) MEASUREMENT OF ALKALI RESERVE: (HCO3) = 24mEq/L -4) MEASUREMENT OF BUFFER BASE: -5) ANION GAP MEASUREMENT: CLINICAL EVALUATION OF ACID BASE BALANCE: • MEASUREMENT OF BUFFER BASE: • It is conc of anion component of buffers in body fluid. It includes HCO3 conc & conc of protein anions. • Normally buffer base is 48mEq/L. • Out of this, HCO3 is 24 & remaining is Hb (mainly protein anions). • We can also evaluate acid base balance by acid base nomograms. It also determines type of acid base disturbance & its severity. • ANION GAP MEASUREMENT: It is the difference between conc of cations other than Na & conc of anions other than HCO3 & Cl. ANION GAP MEASUREMENT: • [ANIONS] = [CATIONS] • [MEASURED ANIONS] + [UNMEASURED ANIONS] = [MEASURED CATIONS] + [UNMEASURED CATIONS] • [Cl-] + [HCO3-] + [UNMEASURED ANIONS] = • [Na+] + [UNMEASURED CATIONS] • [UNMEASURED ANIONS – UNMEASURED CATIONS = • [Na+] – [Cl- + HCO3-] ANIONS OTHER THAN HCO3 & Cl: • Protein anions, PO4, SO4 & LACTATE. • Difference between these 2 concs is called ANION GAP. • Anion gap is increased, when conc of cations decreases or anions are increased,e.g, incresed albumin, SO4,PO4,LACTATE & PYRUVATE. ANION GAP IS INCREASED IN: • Metabolic acidosis due to ketoacidosis & lactacidosis like in uncontrolled DM (ketoacidosis) & in severe hypoxia (lactacidosis). ANION GAP IS NOT INCREASED IN: • Hyperchloremic acidosis, which may be due to CA Inhibitors (acetazolamide) or ingestion of large amount of NH4Cl. 1) RESPIRATORY ACIDOSIS: • Here PCO2 in arterial blood increases, ratio between salt & acid falls, so pH decreases to produce resp acidosis. • CAUSES: Decreased rate of pulm vent. due to damage to resp centre or resp centre depression by drugs like morphine or disease of resp centre. Resp muscle paralysis, airway obstruction, pulm fibrosis, pneumothorax & pleural effusion. • In resp acidosis, cause is in resp system. • COMPENSATORY MECHANISMS: (from outside resp system) • 1) Various non-HCO3 buffers, take up or buffer H ion to produce HCO3 ion. When there is increased PCO2, there is more H2CO3. So non-HCO3 buffers will take up H ion from H2CO3 & left behind is HCO3. • 2) Renal compensation: • In renal tubules, there is more H ion secretion & more NH3 secretion. More HCO3 reabsorption or regeneration. More titrable acidity of urine. • As a result of renal compensation, HCO3 will increase. • Ratio of HCO3/PCO2 conc will increase back to normal pH will increase back to normal. • In compensated cases of resp acidosis, there is some metabolic alkalosis because HCO3 is increased. 2) RESPIRATORY ALKALOSIS: • Here PCO2 in arterial blood decreases. So ratio between salt/acid conc is increased, so pH increases resp alkalosis. CAUSES: Hyper ventilation: • Voluntary • Hysteria / psychoneurosis • Resp centre stimulation in salicylate poisoning & nikethamide (resp stimulant) • At high altitude. • COMPENSATION OF RESP ALKALOSIS: • 1) By protons donated by various buffers in body fluids (some compensation). • 2) Main compensation is through kidneys. • In kidney, no H ion secretion, no NH3 secretion, HCO3 is not reabsorbed, it is lost in urine in large amount. H ions are produced in tubular cells which are added to ECF. So urine will be highly alkaline.