Ethers
advertisement
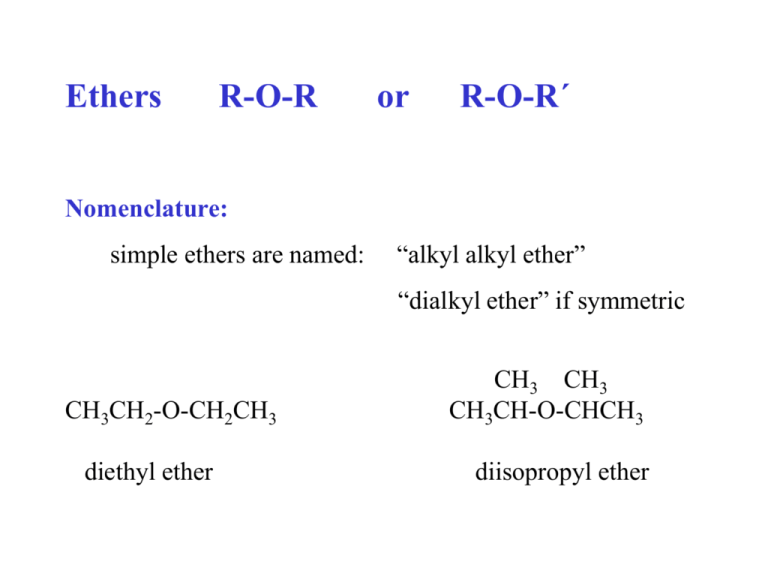
Ethers R-O-R or R-O-R´ Nomenclature: simple ethers are named: “alkyl alkyl ether” “dialkyl ether” if symmetric CH3CH2-O-CH2CH3 diethyl ether CH3 CH3 CH3CH-O-CHCH3 diisopropyl ether CH3-O-C(CH3)3 tert-butyl methyl ether (MTBE) If complex, the ether part is named as an “alkoxy” group: CH3-O- = methoxy CH3CH2-O- = ethoxy, etc. CH3-O-CH2CH2CH2-O-CH3 1,3-dimethoxypropane HO-CH2CH2-O-CH2CH3 2-ethoxyethanol Physical properties: R : : O R' oxygen is sp3 hybridized, bond angle ~ 109.5o ethers are polar; no hydrogen bonding mp/bp moderate water insoluble Diethyl ether = very important organic solvent, polar, water insoluble, bp = 35o. Very flammable & forms explosive peroxides. Industrial synthesis for diethyl ether: 2 CH3CH2-OH + H2SO4, 140oC CH3CH2-O-CH2CH3 + H2O Not generally useful for syntheses of ethers in the lab: a) Only symmetric ethers can be made this way. b) Conditions are very compound specific ( at 180o ethanol would yield ethylene instead of the ether) Synthesis of ethers 1. Williamson Synthesis. R-O-Na+ + R´-X R´-X should be CH3 or 1o SN2 mechanism R-O-R´ + NaX Mechanism for the Williamson Synthesis = SN2 RDS R O + R' X R´-X should be CH3 or 1o R O R' + X R-OH + Na R-O-Na+ R-O-R´ R´-OH + HX R´-X (CH3)2CH-OH + Na (CH3)2CH-O-Na+ + CH3CH2CH2-OH + HBr CH3CH2CH2-Br CH3CH2CH2-O-CH(CH3)2 isopropyl n-propyl ether note: the alkyl halide is primary! CH3CH2CH2-OH + Na CH3CH2CH2-ONa + CH3CH2CH2-O-CH(CH3)2 (CH3)2CH-OH + HBr (CH3)2CH-Br 2o alkene The product of this attempted Williamson Synthesis using a secondary alkyl halide results not in the desired ether but in an alkene! The alkyl halide in a Williamson Synthesis must beCH3 or 1o! Synthesis of di-tert-butyl ether? CH3 CH3 CH3C-O-CCH3 CH3 CH3 Cannot be made via the Williamson Synthesis. The alkyl halide would be 3o. 2. Alkoxymercuration-demercuration A way to make ethers that cannot be made via the Williamson Synthesis. (later) R-H R-X R-OH Metals NR NR NR NR some NR NR Oxid. NR NR NR Reduc. NR NR NR Halogens NR NR NR NR Acids Bases R-O-R Reactions, ethers: 1. Acid cleavage. R-O-R´ + (conc) HX, heat CH3CH2-O-CH2CH3 + HBr, heat R-X + R´-X 2 CH3CH2-Br Mechanism 1) CH3CH2 O CH2CH3 + HX SN2 H O CH2CH3 2) X 3) CH3CH2 OH + HX + CH3CH2 CH3CH2 CH3CH2 X + HO CH2CH3 CH3CH2 OH2 SN2 4) CH3CH2 OH2 + X H O CH2CH3 + X CH3CH2 X + X + H2O Alkanes Nomenclature Syntheses 1. 2. reduction of an alkyl halide a) hydrolysis of a Grignard reagent b) with an active metal and acid 3. Corey-House Synthesis Reactions 1. halogenation 2. combustion (oxidation) 3. pyrolysis (cracking) Alkyl halides: nomenclature syntheses: 1. from alcohols a) HX b) PX3 2. halogenation of certain alkanes 3. 4. 5. halide exchange for iodide reactions: 1. nucleophilic substitution 2. 3. formation of Grignard reagent 4. reduction Alcohols: nomenclature syntheses later reactions 1. HX 2. PX3 3. 4. as acids 5. ester formation 6. oxidation Ethers nomenclature syntheses 1. Williamson Synthesis 2. reactions 1. acid cleavage Mechanisms: Free radical substitution SN2 SN1 Memorize (all steps, curved arrow formalism, RDS) and know which reactions go by these mechanisms!








