Ethers - Home - KSU Faculty Member websites
advertisement

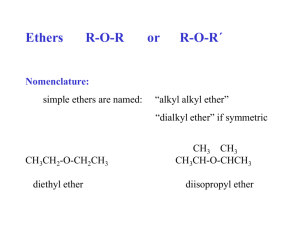
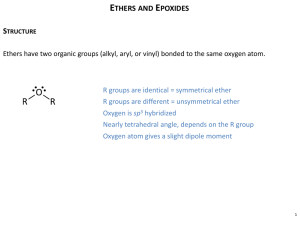
Chem 145 Ethers and Epoxides Chapter 8 1434-1435 2013-2014 2nd semester Chapter’s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods of preparation of ethers A- Dehydration of Alcohols. B- Williamson Synthesis. Reactions of ethers Cyclic Ethers; “Epoxides” Definition General methods of preparation of Epoxides Reaction of Epoxides. Definition Ether is a class of organic compounds that contain an ether group — (an oxygen atom connected to R two alkyl or aryl groups) — of general formula R–O–R’. O R' Classification of Ethers (I) Aliphatic Ethers (II) Aromatic Ethers Aliphatic ethers are those in which R and R' are both alkyl groups. Aromatic ethers are those in which either one or both R and R' are aryl groups. Examples: Example: CH3CH2CH2CH2 O Butylmethylether CH3 CH3 O O Methyl phenylether C6H5 O Diphenylether C6H5 Nomenclature: A. Common Names. In the Common system the ethers are named according to the alkyl group bonded to the oxygen atoms. The two-alkyl groups bonded to the functional group (- O -) are written alphabetically followed by the word ether. Examples CH3 - O - C2H5 Ethyl methyl ether O CH2CH3 Ethyl phenyl ether O O Diethylether Tert-butylmethyl ether B. IUPAC System For Ethers The names for ethers are based on the alkane name of the longest chain attached to the oxygen. The shorter alkyl group and the oxygen are named as an alkoxy group attached to the longer alkane. methoxy They are named as alkoxyalkanes. propane Numbering the longer alkane gives CH3—O—CH2—CH2—CH3 alkoxy group Alkyl Group CH3CH3CH2(CH3)2CH(CH3)3CC6H5- Name Methyl Ethyl Isopropyl tert-Butyl Phenyl 1-methoxypropane. Alkoxy Group CH3OCH3CH2O(CH3)2CHO(CH3)3COC6H5O- Name Methoxy Ethoxy Isopropoxy tert-Butoxy Phenoxy Examples O CH3 CH3 (a) (b) CH3CHCH2 O 3 2 CH3 1 1-Methoxy-2-methyl propane Methoxy cylohexane O CH2CH3 H3C (c) CH3CH2CH2 O CH2CH2CH2CH3 1 Propoxy butane 2 3 4 (d) 1-Ethoxy-1-methyl Cyclohexane O O 5-ethoxy-2-heptene 1-phenoxy-1-propene O O Common: Diphenylether Methylphenylether Anisol IUPAC: Phenoxy benzene Methoxy benzene Exercise: 1- Give the name of the following molecules (IUPAC & Common) O 2- Draw the correct structure for the following A) Methyl vinyl ether B) Methoxy hexane Physical Properties 1. Solubility of Ethers • Ethers containing up to 3 carbon atoms are soluble in water, due to their hydrogen bond formation with water molecules. Ethers can form hydrogen bonds with water molecules • The solubility decreases with increase in the number of carbon atoms. • The relative increase in the hydrocarbon portion of the molecule decreases the tendency of H-bond formation. 2. Boiling Points of Ethers Ethers have an O atom, but there is no H attached. Thus, hydrogen bonds cannot form between ether molecules. O R’ R Ether molecules cannot form hydrogen bonds with other ether molecules. CH3CH2CH2CH3 CH3 O CH2CH3 CH3CH2CH2OH Butane Methoxyethane 1-Propanol (butane) (ethyl methyl ether) (Propyl alcohol) M.W. = 58 b.p. = - 0.5°C M.W. = 60 b.p. = 7.9 °C M.W. = 60 b.p. = 97.2°C General methods of preparation of ethers A- Dehydration of Alcohols.(only symmetric ethers) • This method is used for industrially preparation for symmetric ethers. • In the presence of acid, two molecules of an alcohol may lose water to form an ether. General Equation R' OH + R" OH H+ heat /140°C R' O R" + H2O Examples 2 CH3OH 2 CH3CH2OH H2SO4 140°C H2SO4 140°C CH3 O CH3 + CH3CH2 O CH2CH3 H2O + H2O B- Williamson Synthesis. The reaction of a sodium alkoxide; RONa or a sodium phenoxide; ArONa with an alkyl halide to form an ether is known as the Williamson synthesis. - It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. - The reaction involves nucleophilic substitution of an alkoxide ion for a halide ion. General Equations R O- Na+ + Sodium alkoxide Ar O- Na+ + R' X Alkyl halide R' X Sodium phenoxide Alkyl halide R O R' + NaX Alkyl ether Ar O R' + NaX Aryl ether Examples CH3CH2 O Na CH3CH2Br CH3CH2 O CH2CH3 O Na O CH3 CH3I ! NaBr Na I Note that The alkoxide is commonly made by adding Na or K to the alcohol Examples + O Na OH Na OCH2CH3 CH3CH2 Br + sodium cyclohexanol OH cyclohexyloxide NaBr ethoxycyclohexane OCH3 1) Na 2) CH3-I 3,3-dimethyl-2-pentanol 2-ethoxy-3,3-dimethypentane Reactions of Ethers Ethers undergo just one kind of basic chemical reaction: cleavage by acids. Cleavage of Ethers by Acides Ethers are cleaved by HX to an alcohol and a haloalkane. General Equation R O R' + HX Ether Heat R X + Alkyl halide Conc. acid R' OH alcohol Specific Example CH3 O CH3 + HBr Dimethyl ether Heat Hydrogen bromide CH3 Br + HO CH3 Methyl bromide Methyl alcohol Note: If two or more equivalents of acid are used further dehydration can occur on formed alcohols which may react further to form a second mole of alkyl halide. Example CH3CH2OCH2CH3 Diethyl ether + 2 HBr Heat Excess Hydrogen bromide 2 CH3CH2Br + H2O Ethyl bromide Cyclic Ethers; “Epoxides” Epoxides Definition O An epoxide Epoxides or Cyclic ethers Epoxides are cyclic ethers in which the ether oxygen is part of a threemembered. The simplest and the most important epoxide is ethylene oxide. O O H C H H H H H Ethylene oxide C H H General methods of preparation of Epoxides Peroxide Epoxidation Epoxides are often prepared from reacting with organic peroxy acids (“peracids”) ex; CH3C(O)OOH in a process called epoxidation. General equation O RCH CHR' + R"CO OH MCPBA RCH CHR' O Peroxy acid Note that: Epoxide Cl MCPBA= CO3H + R''COOH Example CH3 CH CH2 phCO3H H3C CH CH2 O propeneoxide propene MCPBA CH2Cl2 O Reaction of Epoxides Ring-opening reactions of epoxides: • Epoxides are highly strained and easily undergo ring-opening reactions under both acidic and basic conditions. • For the same reason epoxides have the tendecy to open up its ring. Thus oxygen atom combines with the reactive hydrogen atom of vairous compounds to form a hydroxy group, -OH, as shown in the next slide. General Equation CH 2 + H Y CH 2 O Ethylene oxide CH2 CH2 OH Y Addition Product Reaction of Epoxides. H2O/H CH3OH/H CH2 CH2 OH OH CH2 CH2 OH OCH3 1,2-Ethandiol 2-Methoxyethanol O CH2 CH2 + HX/H CH2 CH2 OH X RMgX/dryether CH2 CH2 H3O OH R Ethylene oxide Ethylene halohydrin 2-Halo ethanol Alkyl alcohol Thank you For your attention