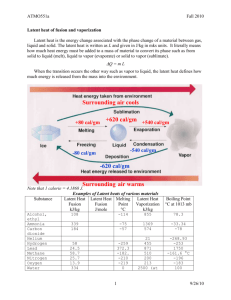
ppt file for this lecture (click to file)
advertisement

The Sea Around Us Lecture 3: Properties of Water: The Wonder Substance Today’s music: Bill Nye Water Cycle Jump! From: A Gentle Introduction to Water www.chem1.com/acad/sci/aboutwater.html NIGHTWISH - The Islander Ron Pope - A drop in the ocean The Presets - Girl and the sea Thanks to Hannah P., Kara D. & Daisha M. • Lecture Notes and Review Questions • TA Office Hours (T 10-11 & W 2-3) • On-line Assignment 1 is due tonight by 11pm • Homework 1 due Jan 28 • Cell Phone Recycling • Songs and Questions (Angel) World Ocean Council See link for Internship Program. Florida couple planning for life under the sea http://www.wtsp.com/news/s cience/story.aspx?storyid=16 9031&catid=67 Current progress: underseacolony.com/prime/mainhub_revA.html Read your book anywhere and everywhere! True (ocean) love!!? Today is the first in-class iClicker exercise for credit A) Full credit if you answer 80% or more of the questions B) Bonus points if you get the correct answer for 80% of more of the questions C) If there are 8 questions and you answer at least 7 of them you’ll get full credit (100%) D) If there are 8 questions and you answer at least 7 of them correctly you’ll get a 5% bonus (105%) E) All of the above (this is the correct answer, choose E!) Water in the Oceans --and why we should be concerned about the physical properties of water •Importance to Physical & Biological Processes •Water’s unique physical properties cause a buffering of Earth’s surface and environmental changes, which promotes continuity of life on Earth •The physical properties of water determine how oceans circulate, how heat is transferred by the ocean and atmosphere, and the way waves work. Water Promotes Continuity of Life on Earth Low viscosity •rapid flow to equalize pressure differences Low compressibility •Small change in volume for a change in pressure High heat capacity •cools/warms slowly relative to land •aids in heat retention & transport •minimizes extremes in temperature •helps to maintain uniform body temps High latent heat of evaporation/precipitation •very important for heat transfer between ocean & atmosphere (main source of energy for hurricanes!) High surface tension •allows wind energy to be transmitted to sea surface •allows cells to hold shape --and life to form •controls the behavior of water drops Properties of Water: Surface Tension, Viscosity, Compressibility Viscosity: Is the resistance to shear motion Increases as water cools Is low for water, compared to say, ketchup Low viscosity liquids pour or stir easily Ketchup has high viscosity Water has low viscosity Properties of Water: Surface Tension, Viscosity, Compressibility Compressibility (can you squooosh it?) is: • Very low for water • The change in volume for a change in pressure Think about: In the ocean: pressure increases by 1 atm. (14.5 psi) for every 10 m increase in depth •at 4000 m, a liter of water (at the surface) is only ~ 2% smaller •Sea level would be 37m higher if water were totally incompressible before After Compression Properties of Water: Surface Tension, Viscosity, Compressibility Surface tension or capillary force (highest of all liquids) •cohesion of liquid surface- intermolecular forces •water forms weak "elastic" membrane •increases as water cools •decreases with increasing salt content meniscus Surface tension is what holds water drops together and determines the shape of waves. Ripples and small waves are generated by wind energy and the surface tension of water. Physical Properties of Water. Check out Water Drop at 2000 Frames per Second http://www.flixxy.com/water-drop.htm#.UPQpcIVe8oY%3Cp%3E From Discovery Channel's series 'Time Warp' where MIT scientist and teacher Jeff Lieberman and digital-imaging expert Matt Kearney use the latest in high-speed photography to turn never-before-seen wonders into an experience of beauty and learning. Physical Properties of Water: The Wonder Substance Molecular Structure and Organization •Bonding of hydrogens to oxygen creates a "polar" molecule. Water molecule: • 105° angle • unequal charge distribution: Polar Molecular Structure and Organization: Clustering of water molecules What causes it? •hydrogen bonds (weak interaction ~10% of covalent strength) •This property creates Surface Tension Liquid water; bonds are constantly breaking and reforming •each bond lasts a few trillionths of a second •high proportion of molecules bonded at any instant Water Promotes Continuity of Life on Earth Low viscosity •rapid flow to equalize pressure differences High surface tension •allows wind energy to be transmitted to sea surface •allows cells to hold shape --and life to form •controls the behavior of water drops High heat capacity •cools/warms slowly relative to land •aids in heat retention & transport •minimizes extremes in temperature •helps to maintain uniform body temps High latent heat of evaporation •very important in heat/water transfer in atmosphere Properties of Water Unusual Properties--compare other liquids Heat Capacity or Specific heat: What is it? • Ability of a material to store heat • Heat needed to change the temperature of a given mass of water (1 gram or 1 kilogram) by 1 degree C For Water: heat capacity is: 1 cal per gram per deg. Celsius: 1cal/(gm °C) • 1 calorie is the heat energy needed to raise 1 gm by 1°C • 1 calorie ≈ 4.19 J (heat capacity of water is 4190 J/Kg °C) • Need 41,900 J to raise the temperature of 1 kg (1 liter) of water from 0 to 100° C Heat capacity (high) •only ammonia (NH3) higher •H20 much higher heat capacity than rock or steel 3 phases of materials Heat required to change the temperature (by 1 deg.) of a given mass 4190 J Kg °C -1 Temperature (°C) Heat Capacity Rock & Soil Liquid water Heat input (J/kg or cal/gram) Heat capacity and phase changes: ice (solid) 150 water (liquid) vapor or steam (gas) Vapor 100 vapor+ liquid Liquid water 50 Latent Heat Heat needed to change phase (from solid to liquid, liquid to gas, liquid to solid, etc.) Latent heat of vaporization or condensation 540cal/gm Ice + liquid 0 -50 Ice Latent heat of fusion or melting 80cal/gm -100 0 200 400 600 Heat input (cal/gram) 800 Pepsi Temperature (°C) Heat Capacity Heat required to change a given mass by a given temp. J/kg or cal/gram Lower Heat Capacity Higher Heat Capacity Heat input (J/kg or cal/gram) Latent Heat and Changes of State Latent heat of fusion (or melting) • Heat to form or melt ice (liquid to solid phase) • 333 kJ/kg (80 calories/gram) Latent heat of vaporization (or precipitation) • Heat to vaporize (boil) a liquid or condense liquid from a gas phase • 2260 kJ/kg (540 calories/gram) Evaporation of water from the surface can occur at any temperature. However, it takes more energy to evaporate at low T than to boil off vapor once water reaches 100°C The high heat capacity of water means that it heats up and cools off more slowly than land. Latent heat is a key factor in Hurricane development and sustainability. Properties of Water: Heat Capacity What is heat capacity? Why is it so high for H20? Adding heat to water: • speeds up molecules • break bonds Hence, less warming or less evaporation than expected When heat is removed from water: • bonds form and restructure, material condenses (e.g., gas to liquid) • energy is released via bonds formation Energy release causes heating and thus temperature decrease is smaller than expected Density of Water •Fresh water reaches maximum density at 3.98 °C •Density= 1,000 kg/m3 (1kg/liter) • Density decreases as water is heated above 4°C • At 20 °C, density of pure H2O is 998.23 kg/m3 Density of Fresh Water & Ice •Ice is less dense than water. Ice at 0 °C is 917.0 kg/m3 •Ice has an open hexagonal structure Water molecular structure Ice molecular structure Density of ice is about 91% of liquid water Water Promotes Continuity of Life on Earth Low viscosity •rapid flow to equalize pressure differences High surface tension •allows wind energy to be transmitted to sea surface •allows cells to hold shape --and life to form •controls the behavior of water drops High heat capacity •cools/warms slowly relative to land •aids in heat retention & transport •minimizes extremes in temperature •helps to maintain uniform body temps High latent heat of evaporation •very important in heat/water transfer in atmosphere • Heat is required to change the phase of a substance (for example, ice to water); this is known as latent heat 50 water 0 • If we add heat to water (or ice) the temperature rises, this is known as sensible heat Latent heat Ice Sensible heat -50 • Ice changes to water (melts!) at 0° C Temperature (°C) Heat, Temperature and Changes of Phase 10 Heat (cal, or Joules) 50 Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water Pepsi 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat capacity and phase changes: ice (solid) 150 water (liquid) vapor or steam (gas) Vapor 100 vapor+ liquid Liquid water 50 Latent Heat Heat needed to change phase (from solid to liquid, liquid to gas, liquid to solid, etc.) Latent heat of vaporization or condensation 540cal/gm Ice + liquid 0 -50 Ice Latent heat of fusion or melting 80cal/gm -100 0 200 400 600 Heat input (cal/gram) 800 Density of Water •Fresh water reaches maximum density at 3.98 °C •Density= 1,000 kg/m3 (1kg/liter) • Density decreases as water is heated above 4°C • At 20 °C, density of pure H2O is 998.23 kg/m3