Notes
advertisement

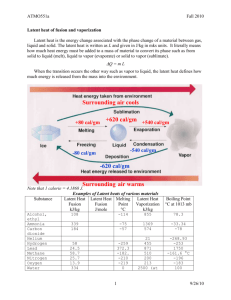
AOM 4346 - BASIC HYDROLOGIC CONCEPTS As discussed last time -- hydrologic cycle describes the continuous circulation of water from land and sea to the atmosphere and back again. The concept is based on mass balance and is simply that water changes state and is transported in a closed system which extends approximately 1 km down into the earth’s crust and 15 km up into the atmosphere. The hydrologic cycle is closed only globally, not on a watershed or continental scale. Thus practicing hydrologists are typically faced with an open system. Also, as we discussed last time, hydrologic phenomena (precipitation, ET, infiltration, groundwater, overland, streamflow) are extremely complex and although quantifiable at lab scale, may never be fully predictable at the watershed scale. Thus we represent them in a simplified way by means of the systems concept. Definition: A hydrologic system is defined as a structure (surface or subsurface) or volume (atmospheric) in space, surrounded by a boundary, that accepts water and other inputs (such as air or heat energy), operates (physical, chemical, biological) on them internally and produces them as outputs. It represents flow paths though the system. Thus we treat the hydrologic cycle as a system whose components are precipitation, evapotranspiration, interception, runoff, infiltration, etc.. We give up the quest to know the precise spatiotemporal water flow patterns within the system and settle instead for knowing total water storage, and spatially averaged water fluxes in and out of the control volume. Example: ET(t) overland flow surface runoff groundwater discharge Q(t) G(t) The basic relations of physical hydrology for this system are derived from fundamental laws of classical physics. Particularly: 1. Conservation of mass (m = mass of water) 2. Conservation of energy (internal energy, kinetic energy and potential energy of the fluid) Conservation of Mass The most useful principal in hydrologic analysis and is required in almost all problems. Stated mathematically dS Q (t ) I (t ) dt For our watershed problem: ppt. surface outflow gw outflow dS P (t ) Q (t ) G (t ) ET (t ) dt These fluxes are spatially integrated over whole control volume -- space is no longer considered an independent variable. Do not consider within control volume processes such as overland flow, gw discharge to stream, within control volume streamflow. open system so there can be a change in storage. If have a closed system (i.e. cycle over whole earth rather than watershed) dS = 0 dt If dS = 0 steady flow problem inflows = outflows: dt P(t ) Q(t ) G (t ) ET Energy Conservation Equation Second fundamental physical law utilized in physical hydrology is the conservation of energy. (energy is neither created or destroyed) Total energy of a system = internal energy + kinetic energy + potential energy extensive property or E Energy per unit mass intensive property = Eu + 1/2 mV2 + mgz = eu + 1/2 V2 + gz Internal Energy (eu) Internal energy is the sum of sensible heat and latent heat. Sensible heat is that part of the internal energy that is proportional to the substance’s temperature temperature changes produce proportional changes in internal energy according to deu = CpdT specific heat of a substance at a constant pressure (1.0 cal/g K for water) (4.2 * 103 J/kg K for water) (0.24 cal/ g K for dry air) Latent heat - Amount of heat exchange required for inducing a phase change per gram of substance without a change in temperature. Usually a function of temperature. For example, 1. liquid water to vapor Le = latent heat of evaporation = 597.3 - 0.57 T cal/g temp in C This is heat absorbed (vaporized by water from surroundings) to break H bonds so evaporation can take place. evaporation always accompanied by transfer of heat out of water body or surroundings to vapor latent heat transfer 2. vapor to liquid water Lc = latent heat of condensation = -597.3 + 0.57 T cal/g C This is heat released to surroundings when H bonds formed during condensation 3. ice to vapor Ls = latent heat of sublimation = 677 - 0.07 T cal/g C This is energy needed to: a) disrupt molecular structure then b) break H bonds At typical atmospheric temp. and pressure on earth, energy required to sublimate ice to vapor generally greater than that required to melt ice through evaporation. Therefore, usually water goes through liquid phase first. 4. ice to liquid Lm = latent heat of melting = 79.7 cal/g = 0.33 * 106 J/kg Energy required to disrupt molecular structure. 5. liquid to ice Lf = latent heat of fusion = -79.7 cal/g = - 0.33 * 106 J/kg Energy released as molecular structure is formed. 1952 J/kg K Internal energy latent heat of fusion = 80 cal/g = 0.33*106 J/kg latent heat of evaporation 539 cal/g at 100C 2.3*106 J/kg at 100C specific heat Cp = 1 cal/g C =4183 J/kg K water vapor ice 2106 J/kg K liquid water 0 Temp C 100 Jumps in curve latent heat transfer to water Slope in curve sensible heat transfer to water In the SW use the latent heat of evaporation for air-conditioning houses water and air is run into evaporative cooler on roofs of houses -- as water evaporates absorbs heat from air. Cooled air is returned to house. Latent heat transfer is the dominant cause of internal energy change for water in most hydrologic applications temperatures usually only change a few degrees C so sensible heat transfer is small.